An ongoing study of monotherapy with iza-bren, a novel antibody-drug conjugate (ADC), has shown median progression free survival (PFS) of 12.5 months in patients with previously treated locally advanced or metastatic EGFR-mutated non-small cell lung cancer (NSCLC). In patients who were chemotherapy naïve but post tyrosine kinase inhibition, the objective response rate (ORR) was 56% after median follow up of 20.5 months. A second study of iza-bren plus osimertinib in the frontline setting has demonstrated a 95% confirmed objective response rate. Neither of the iza-bren studies had reached median overall survival (OS) as of the data reporting cutoff at the end of June 2025.
Iza-bren is a potential first-in-class EGFR-HER3 bispecific ADC linked to a novel topoisomerase I inhibitor payload.
Results of the two phase II trials were previewed Saturday afternoon during the 2025 World Conference on Lung Cancer. The data will also be reviewed and discussed today, Monday, September 8, during an oral abstract session, Optimizing Systemic Therapy: Bridging New and Old. The session will begin at 15:30 CEST in Room 07.
Third-generation EGFR-TKI agents are currently the standard first-line therapy for patients with EGFR-mutated NSCLC, said Wenfeng Fang, MD, PhD, Sun Yat-sen University Cancer Center, Guangzhou, China. However, nearly all patients develop drug resistance. Subsequent therapeutic options are limited with median progression-free survival (PFS) often ranging between 4 to 6 months. There is a huge unmet need to explore new agents and new strategies for EGFR-mutated NSCLC, he said.
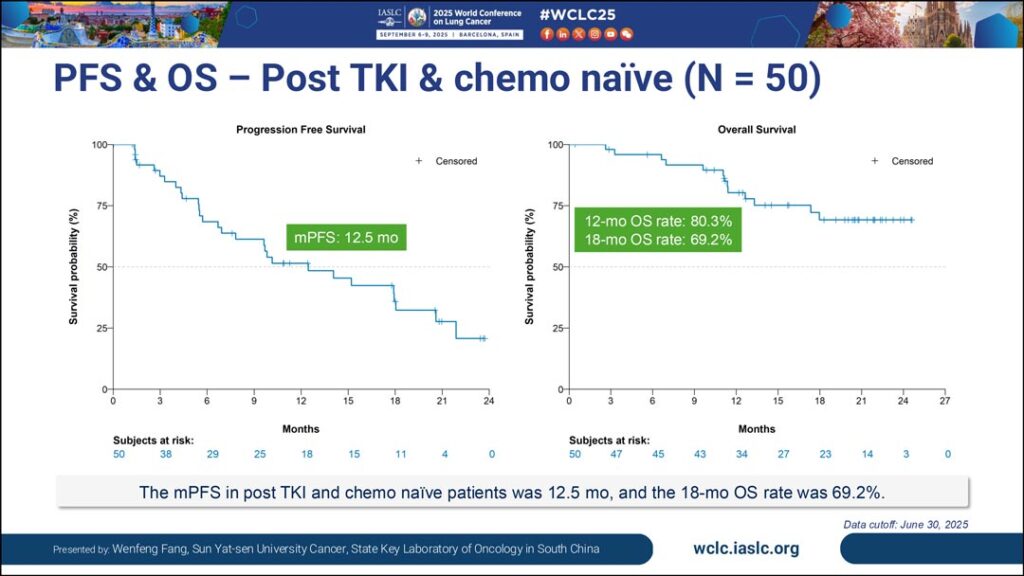
The monotherapy trial included 171 patients with EGFR-mutated NSCLC. Dr. Fang reported results from a subgroup analysis of 50 patients who received a 2.5 mg/kg dose of iza-bren on day 1 and day 8 of every 3-week cycle. In this group, he said they saw an objective response rate (ORR) of 66%, a confirmed ORR of 56%, a median PFS of 12.5 months, and a median duration of response of 13.7 months. Most patients (94%) showed shrinkage of tumors with a median of 38.6% reduction in size. Investigators saw a 12-month OS rate of 80.3%, though median OS was not yet reached.

“To my knowledge, this is the longest PFS that has been reported to date for an ADC,” said Dr. Fang. “This result is very promising.”
The safety profile was as expected from earlier trials with primarily hematologic events observed. The most common treatment-related adverse events (TRAEs) were anemia (90.6%), leukopenia (80.7%), neutropenia (78.4%), and thrombocytopenia (74.3%). The most common non-hematologic TRAEs were nausea, alopecia, and asthenia. Dr. Fang reported 1.2% of patients discontinued treatment for TRAEs and said there were no treatment-related fatalities.
Iza-bren showed similar promising results when used in combination with osimertinib as first-line treatment for EGFR-mutated NSCLC. Fei Zhou, MD, PhD, Shanghai East Hospital, Shanghai, China, said the confirmed ORR in a cohort of 40 patients treated with 2.5mg/kg was 95% (38 patients) with the remaining two participants still awaiting confirmation of ORR at data cutoff.
The 12-month PFS was 92.1% and the OS rate was 94.8% at 12 months. All of the patients in the 2.5 mg/kg cohort had tumor shrinkage with a median of 56.7% reduction in tumor size. The median follow up was 12.5 months.

Dr. Zhou said there were no new safety signals compared to earlier trials of iza-bren combination therapy. Serious TRAEs, grade 3 or 4, were again predominantly hematologic, primarily leukopenia, neutropenia, or thrombocytopenia. Non-hematologic TRAEs included anemia, nausea, and stomatitis. Dr. Zhou said most TRAEs were managed with standard supportive measures or dose reductions. He said 13% of patients discontinued combination treatment due to adverse events. There were no grade 5 TRAEs.
“These results are remarkable and suggest that this combination could offer a potentially transformative first-line treatment option for EGFR-mutant NSCLC,” Dr. Zhou said. “Importantly, the regimen was also manageable from a safety perspective.”
Phase III registration trials for iza-bren monotherapy in EGFR-mutated NSCLC after progression on third generation TKI therapy and as first-line therapy in combination with osimertinib are underway in China.

Optimizing Systemic Therapy: Bridging New and Old
15:30 Central European Summer Time (CEST) • Room 07
In addition to data on iza-bren, an oral abstract session Monday afternoon will also explore data on datopotamab deruxtecan and bevacizumab plus chemo. Learn More










