Lung cancer in patients with no smoking history is the fifth most common cause of cancer-related deaths worldwide. Experts predict its incidence will likely increase during the next 10 to 20 years.
Traditionally, lung cancer screening guidelines have relied heavily on current or prior smoking history to determine eligibility for screening. The Female Asian Nonsmoker Screening Study (FANSS) was designed to evaluate the feasibility and efficacy of lung cancer screening in Asian women with no smoking history.
Elaine Shum, MD, assistant professor and thoracic medical oncologist, NYU Perlmutter Cancer Center, presented findings from the FANSS study during the session Global Lung Cancer Screening Strategies at the 2025 World Conference on Lung Cancer (WCLC).

Global Lung Cancer Screening Strategies
Registered WCLC 2025 attendees can still watch Elaine Shum, MD, and other experts share international perspectives on lung cancer screening initiatives through on-demand access. LEARN MORE
Study Design and Criteria
The trial enrolled 1,000 participants, specifically women of Asian descent aged 40 to 74 years with no smoking history.
Participants underwent a shared decision-making discussion addressing the risks and benefits of lung cancer screening before undergoing a low-dose computed tomography (LDCT) scan, followed by monitoring according to Lung-RADS guidelines. Additionally, participants provided plasma samples for circulating tumor DNA (ctDNA) analyses.
The primary objective was to develop a database containing clinical, demographic, and radiographic data on Asian women with no smoking history who underwent LDCT to assess the feasibility of lung cancer screening. Secondary objectives included:
- Lung cancer detection rate
- Incidence of thyroid nodules
- Incidence of coronary artery disease
- Lung cancer detection rate using plasma-based ctDNA
Key Findings
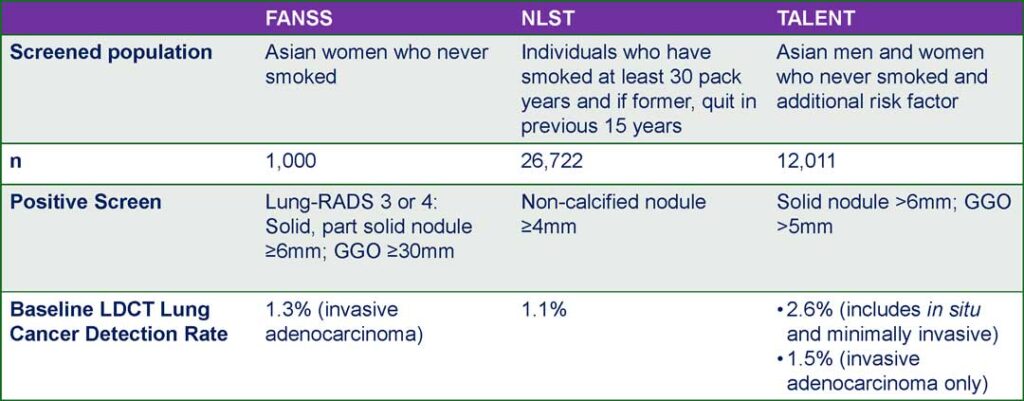
Based on baseline LDCT scans, the FANSS lung cancer detection rate was 1.3%, comparable to the TALENT study’s rate for high-risk populations with no smoking history in Taiwan (1.5%)1 and higher than the rates from the National Lung Screening Trial (1.1%)2 and the NELSON study (0.9%).3
Among the 1,000 LDCT scans performed, most participants had Lung-RADS scores of 1 (38.8%) or 2 (52.1%). Positive LDCT scans, defined as Lung-RADS 3 or 4, accounted for 6.9% of all participants.
Dr. Shum noted that 14 participants had Lung-RADS 3 or 4, findings highly suspicious for malignancy. “They remain in imaging follow-up at this time, perhaps suggesting that our lung cancer detection rate at baseline might be an underestimate,” she said.
The findings revealed that among the cases detected, 69.2% were stage IA, 15.4% were stage IIB, and 15.4% were stage IIIB or IIIC. All detected cases were adenocarcinomas and harbored a driver mutation (EGFR or HER2). Additionally, most participants reported having no family history of lung cancer (85% vs. 15%).
Dr. Shum highlighted two significant cancers detected in the study after a third LDCT.
The first participant was classified as Lung-RADS 2 during the first 2 years of the study.
This was later upgraded to Lung-RADS 4B due to a growing nodule in the left lower lobe. Ultimately, the participant was diagnosed with stage IA lung adenocarcinoma harboring an EGFR L858R mutation.
The other participant, who had a strong family history of EGFR-mutated lung cancer, was classified as Lung-RADS 2 throughout the course of the study. However, due to a persistent ground glass opacity (GGO), they underwent surgery and were diagnosed with adenocarcinoma in situ.
Dr. Shum noted that the criteria for a positive screen can vary between studies. For example, in the TALENT study, a solid nodule larger than 6 mm or a GGO greater than 5 mm was considered positive.1
In contrast, FANSS followed Lung-RADS criteria, where categories 3 or 4 are defined as a solid or part-solid nodule measuring 6 mm or greater, or a GGO larger than 30 mm (3 cm).
“This is something we take into account when we think about our lung cancer detection rates, given the different management guidelines that exist between the US and Asia,” Dr. Shum said.
The FANSS trial is the largest lung cancer screening program in the United States dedicated to a population with no smoking history to date, and it demonstrates the program’s feasibility. All detected lung cancers harbored driver mutations (EGFR or HER2), highlighting the distinct biology of lung cancer in non-smoking populations.
“The development of specific screening and management guidelines for screening this population is necessary,” Dr. Shum said. “As lung cancer incidence continues to rise, expanding screening to individuals with no smoking history certainly warrants identifying other high-risk groups who may benefit.”
References
- 1. Chang GC, Chiu CH, Yu CJ, et al. Low-dose CT screening among never-smokers with or without a family history of lung cancer in Taiwan: a prospective cohort study. Lancet Respir Med. 2024;12(2):141-152. doi:10.1016/S2213-2600(23)00338-7
- 2. National Lung Screening Trial Research Team, Aberle DR, Berg CD, et al. The National Lung Screening Trial: overview and study design. Radiology. 2011;258(1):243-253. doi:10.1148/radiol.10091808
- 3. Ru Zhao Y, Xie X, de Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. NELSON lung cancer screening study. Cancer Imaging. 2011;11 Spec No A(1A):S79-S84. Published 2011 Oct 3. doi:10.1102/1470-7330.2011.9020










