After years of work and the recent publication of the updated IASLC Staging Manual in Thoracic Oncology, the 9th edition of the TNM Classification of Lung Cancer has officially replaced all older editions and manuals, which are now outdated, and, as a result, may lead to incorrect TNM staging. To further educate the profession on the changes in the 9th edition, an international discussion of the new recommendations and other staging-related topics took place during the 2024 World Conference on Lung Cancer.

Before going into the details of the updated TNM rules, Hisao Asamura, MD, Professor and Chief of Tokyo Dental College Ichikawa General Hospital, Chiba, Japan, and Professor of Surgery and Chief of the Division of Thoracic Surgery, Keio University School of Medicine, Tokyo, said he wanted to make something clear.
“The first question is ‘what is the stage of cancer?’ How many people in this room can answer this question precisely?” he asked “The answer is the anatomic extent of the disease. Anatomic extent. This is key. Please remember this before going into the details.”
Dr. Asamura, who chaired the committee to revise the staging guidelines, highlighted the key changes clinicians will see. He was joined by several other experts to discuss wide-ranging topics in staging during the session, Lung Cancer Staging: Updates, Limitations, Approach, and Guidelines.
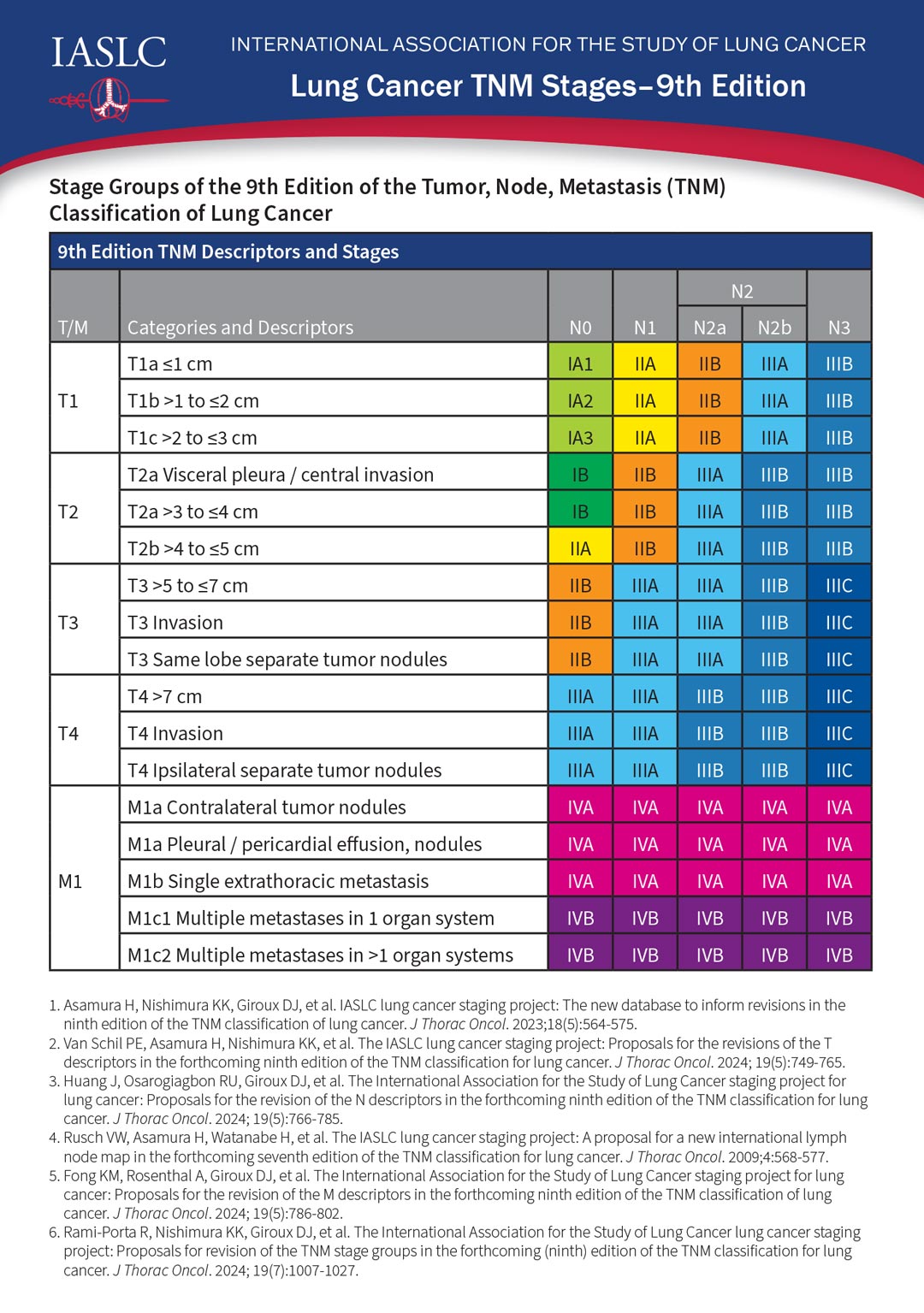
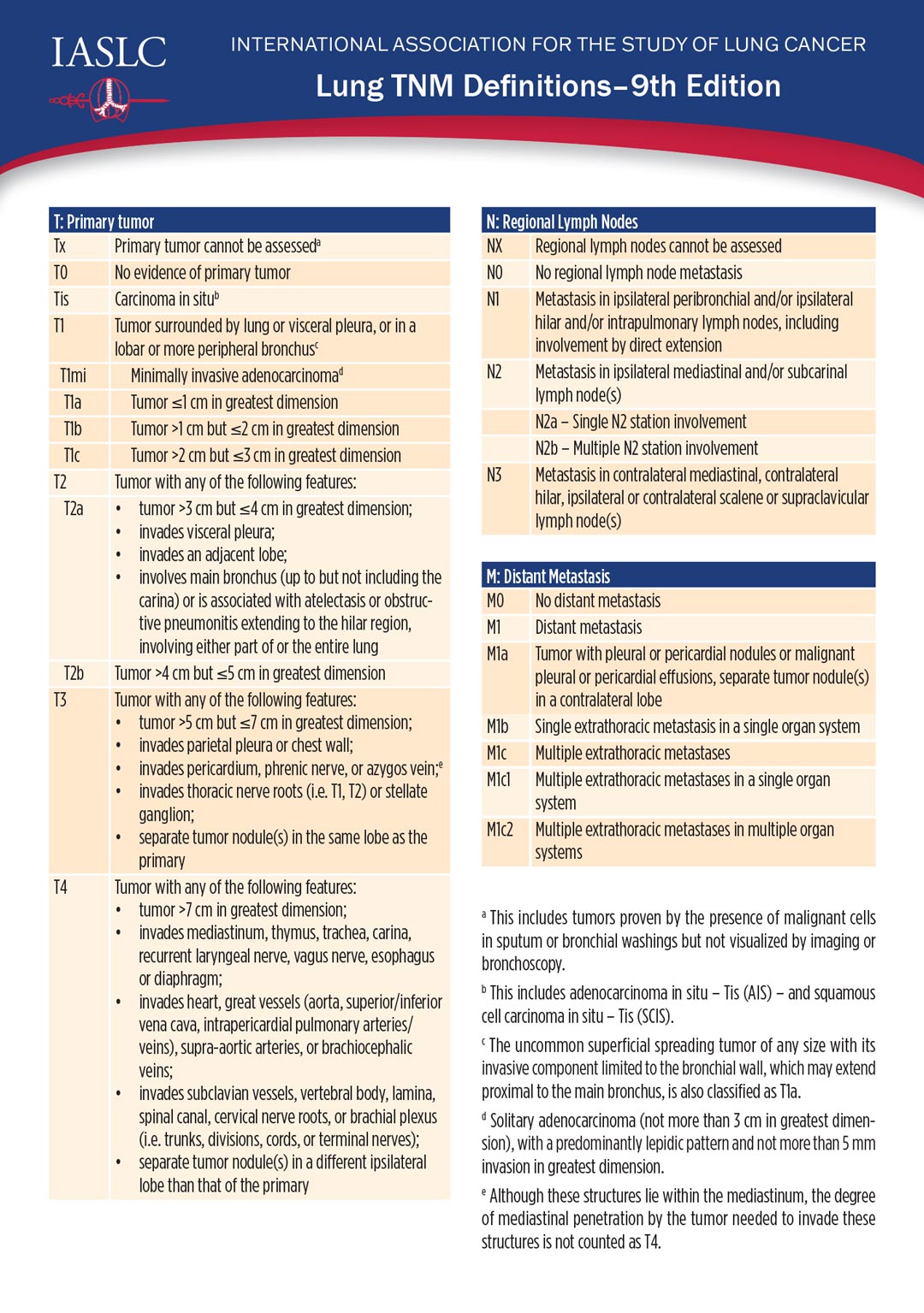
No changes were made to the “T”—or tumor—staging criteria, however, the 9th edition includes updated criteria for the “N”—or nodal status—and for the “M”—metastasis.
The N2 category has been subdivided into N2a and N2b, Dr. Asamura said. N2a denotes metastasis limited to a single ipsilateral mediastinal station. N2b denotes metastases in multiple mediastinal stations.
The M1c category was likewise subdivided into M1c1 and M1c2. M1c1 denotes multiple extrathoracic metastases in a single organ system, and M1c2 denotes multiple extrathoracic metastases in two or more organ systems.
And while the 9th edition was just completed, preparation has already begun on the 10th edition; Dr. Asamura said much more significant changes are likely with that edition.
As he noted in the beginning of his talk, stage has traditionally been an anatomic description. However, growing evidence supports the inclusion of molecular biomarkers and other non-anatomic factors in staging, which includes tobacco use, he said.
“About a fifth of lung cancer patients are using tobacco at the time of diagnosis and about half of those continue to use tobacco afterward,” said Lawson Eng, MD, SM, FRCPC, a medical oncologist at the Princess Margaret Cancer Center and an Assistant Professor in the Department of Medicine at the University of Toronto. “In a systematic review of more than 4 decades of data and 10,000 patients, quitting smoking improves overall survival for patients with non-small cell and small-cell lung cancer by about 25% to 30%.”
Tobacco can impact drug metabolism, enzymes, and pharmacokinetics, as well as response to targeted therapy and immune checkpoint inhibitor therapy.
“Given how important tobacco is on lung cancer outcomes, we should definitely consider this potential factor in lung cancer staging,” Dr. Eng said.
Technology is also changing staging with new advances in functional and metabolic MRI overcoming the limitations of CT scans and other imaging modalities. The Fleischner Society now recommends the clinical use of MRI for common lung disorders, including lung cancer evaluation.
While MRI does not affect T staging, it may improve N staging. A recent development—diffusion-weighted imaging—could display significant differences between malignant and benign nodes, Dr. Eng said.
Whole-body MRI may also improve M staging by highlighting extrathoracic metastases more effectively than a standard CT.
“MRI enables identification, staging, and follow-up of lung cancer,” said Edith Marom, MD, Professor of Diagnostic Radiology and Head of Thoracic Imaging at the Chaim Sheba Medical Center, an affiliate of Tel Aviv University, Tel Aviv, Israel. “It really depends on the environment in which you work, what is available, what the insurance company is willing to pay, and how much we push for it.”
New approaches to bronchoscopy could also improve diagnosis and mediastinal staging. Percutaneous core needle biopsy is the traditional approach, but endobronchial ultrasound (EBUS) and robotic-assisted bronchoscopy (RAB) allow for concomitant N1 and N2 lymph node assessment and are less invasive.
While RAB is not always available or affordable, EBUS is used globally. A trial of radial EBUS with ROSE—rapid on-site evaluation (of biopsy tissue)—produced a diagnostic yield of 88%. This was similar to linear EBUS, according to Lucía Viola, MD, Head of Thoracic Oncology Service at the Colombian Pneumological Foundation in Bogotá, Colombia.
“Tissue is still the issue,” Dr. Viola said. “The bronchoscopic approach allows diagnosis and staging in one single procedure. For us, ROSE is essential and has become our standard of care.”
The traditional standard has been a linear process: Imaging and biopsy for diagnosis, non-invasive staging, and invasive staging with tissue samples.
“Perhaps we should be looking at that differently,” suggested George Eapen, MD, Professor of Pulmonary Medicine at the University of Texas MD Anderson Cancer Center.
Dr. Eapen recommended a parallel approach—obtaining diagnostic and staging information at the same time to reduce procedures and complications—which MD Anderson adopted when it began the clinical use of RAB in 2021.
All procedures are done under general anesthesia with 3D imaging and a ROSE cytotechnologist with telepathology capability. Three years and 1,655 patients later, Dr. Eapen reported a median nodule size of around 1.8cm, an 85% diagnostic yield, and just 1% pneumothorax compared to a historical rate of nearly 2%.
“We are big believers in ROSE,” Dr. Eapen said. “As far as I’m concerned, ROSE is recommended to ensure that you’re obtaining adequate tissue to perform molecular markers and optimizing the handling of the specimen in coordination with your pathologist who can decide which test to prioritize.”

From the Editor, Corey J. Langer, MD, FACP
While welcome and necessary, further refinements in the staging system risk making the process overly complicated and unwieldy. Assigning stage is no longer intuitive. It requires reference to staging cards that incorporate all of the nuances. As for new technology, this poses major challenges globally, particularly in resource-constrained nations where even CT and PET imaging is difficult to access. Although the Fleischner Society now “recommends the clinical use of MRI for common lung disorders, including lung cancer evaluation,” this is scarcely mainstream, even at most academic centers. Costs may well prove prohibitive, and it is not yet clear if such technology will enhance therapeutic decision-making.