
IMpower010 is the first phase III adjuvant cancer immunotherapy study to demonstrate a significant disease-free survival (DFS) benefit for early-stage NSCLC,1,2 leading to approval of atezolizumab in the US, the European Union, and other countries following adjuvant platinum-based chemotherapy for completely resected stage II-IIIA NSCLC patients with PD-L1 expression ≥1% in the US group and for >50% population in the EU.3,4
In previous reports from a prospective, phase III randomized clinical trial, atezolizumab, yielded a statistically significant DFS improvement compared to best supportive care in patients with PD-L1 expression ≥1% and stage II-IIIA NSCLC. The greatest DFS benefit was seen in patients with PD-L1 expression ≥50%.1 No new or unexpected safety signals were identified, and the safety profile of atezolizumab was consistent with prior experience.
In this subgroup analysis, authors investigated DFS and OS outcomes by surgery type in those patients with PD-L1 ≥50% and stage II-IIIA, excluding those with known EGFR/ALK+ disease.
As previously reported, this group of patients realized a 57% reduction in relapse or death after a median follow up of 32 months and a 58% overall survival benefit after a median follow up of 45 months compared to best supportive care in this setting.5,6
Baseline characteristics of the patients were balanced between the intervention and best supportive care groups. In addition, they were consistent with those in the intention-to-treat (ITT) population.
In patients with high PD-L1 expression, the most frequent procedure was lobectomy (74%-75%). Pneumonectomies accounted for 17%-18% of the total. These figures are consistent with those observed in the ITT population.
We found that in stage II-IIIA patients with PD-L1 ≥50% (excluding EGFR/ALK+ patients), the favorable DFS and OS HRs were mainly driven by the lobectomy subgroup. (See Figs. 1-2) The hazard ratio for DFS in the lobectomy subgroup was 0.34 and the hazard ratio for OS in the lobectomy group was 0.31.
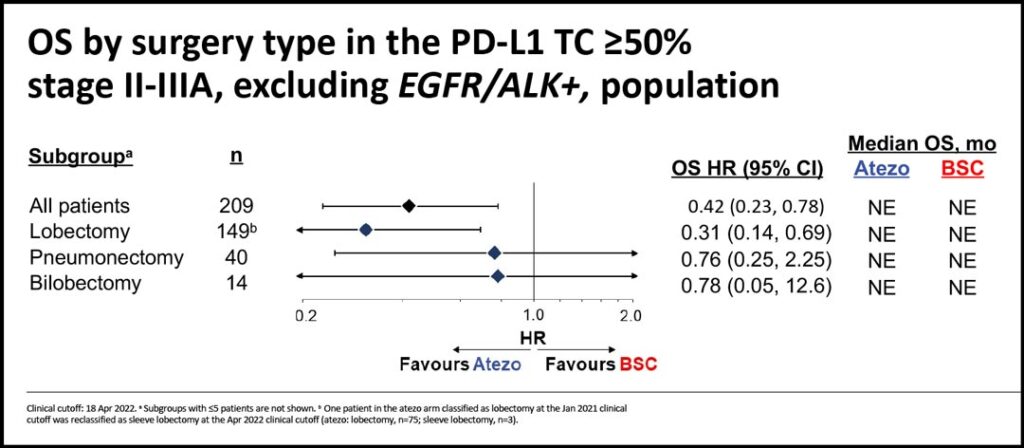
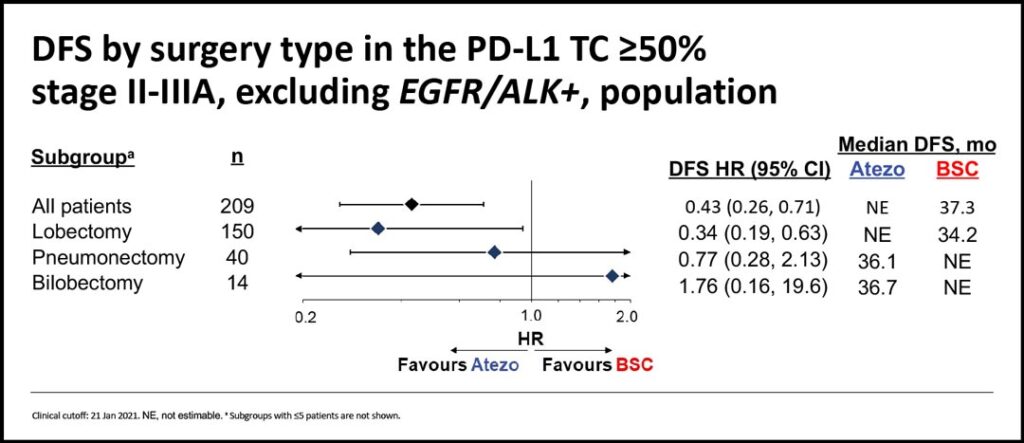
Considering the small numbers of pneumonectomies and bilobectomies, the same benefits were not observed in those undergoing larger procedures.
Pneumonectomies/bilobectomies and lobectomies have previously been shown to result in the same adverse events profiles, with no increase of adverse events in the procedures larger than lobectomies. In addition, the occurrence of adverse events appeared balanced between the intervention and the best supportive care groups in all procedures.7
In conclusion, despite limited patient numbers across surgery types, efficacy outcomes in stage II-IIIA NSCLC patients with PD-L1 expression ≥50% without known EGFR/ALK alterations favored atezolizumab compared to best supportive care and appeared to be most prominent in the lobectomy subgroup. Adjuvant atezolizumab was well-tolerated, and no new safety signals were identified in patients who underwent pneumonectomy or bilobectomy.
These exploratory data support the use of adjuvant atezolizumab after complete resection and platinum-based chemotherapy in stage II-IIIA NSCLC patients with PD-L1 ≥50% without EGFR/ALK alterations.
References
- 1. Felip E, et al. Lancet 2021;398:1344-57;
- 2. Wakelee HA, et al. ASCO 2021. Abstract 8500;
- 3. TECENTRIQ (atezolizumab). Prescribing information. Genentech Inc; 2022;
- 4. TECENTRIQ (atezolizumab). Summary of product characteristics. Roche Registration GmbH; 2022
- 5. Wakelee H, et al. J Thorac Oncol 2022;17(suppl 2):PL03.09;
- 6. Felip E, et al. Ann Oncol 2022;33(suppl 2):S71.
- 7. Lee JM, et al. J Thorac Cardiovasc Surg. 2023 Jan 21:S0022-5223(23)00080-6. doi:10.1016/j.jtcvs.2023.01.012










