In 2020 the U.S. Food and Drug Administration (FDA) launched Project Patient Voice, an online platform for patients and caregivers along with their health care providers to look at patient-reported symptom data from cancer clinical trials.
This new website, how it functions, and how it might be improved was discussed in July at the FDA-ASCO Public Workshop: 2020 Clinical Outcome Assessments in Cancer Clinical Trials Fifth Annual Workshop.
“Because there are limitations for space in a product’s label it is difficult to communicate patient experience data adequately,” said one of the session moderators, Janice Kim, PharmD, MS. “Additionally when patient-reported outcome data are submitted to the FDA for review heterogeneity exists in analysis and presentation of this data.”
The hope for Project Patient Voice is that it will address these issues and be able to provide consistent analytic presentation for this important information, Dr. Kim said.
Pilot Project
Project Patient Voice was launched with data contributed by AstraZeneca’s AURA3 trial. The website will be expanded in the future to include other clinical trials supporting FDA approval of a specific cancer drug.
AURA3 is a phase III randomized trial comparing osimertinib (Tagrisso) with platinum-based chemotherapy in patients with EGFR-mutant locally advanced or metastatic non–small cell lung cancer. As part of the trial, patients reported their symptom experiences via the Patient-Reported Outcomes – Common Terminology Criteria for Adverse Events questionnaire. Symptom experiences were collected at baseline and each week during treatment from patients in both arms of the study.
Data on these outcomes are now posted to Project Patient Voice, which is intended to be used by a health care professional when discussing the potential symptoms related to a cancer and cancer treatment with a patient.
During the FDA-ASCO workshop, Vishal Bhatnagar, MD, associate director for patient outcomes in the Oncology Center of Excellence, and Bellinda King-Kallimanis, PhD, of the FDA’s Center for Drug Evaluation and Research, acted out a role play exercise to demonstrate how they envisioned the website being used.
Dr. King-Kallimanis played the part of a patient recently diagnosed with an advanced malignancy who was getting ready to start therapy with a drug listed on Project Patient Voice. She had questions and concerns about the side effects and symptoms of treatment. Specifically, she asked her clinician if she should be concerned about nausea and whether it might affect a camping trip she has planned in a few months.
Acting as the clinician, Dr. Bhatnagar responded by telling her about Project Patient Voice and pulling up the data about nausea for the drug she is starting. He explained the percentage of patients who experienced nausea while on therapy, and pointed out a chart that displayed the occurrence of nausea on a week-by-week basis. The chart showed that at week 12—when her camping trip would be—the majority of patients like her who had no nausea at baseline had no nausea later on.
Dr. Bhatnagar added that although he could not predict exactly how she would react to the drug, that he hoped this information was helpful.
Detailed Outcome Information
This role play exercise broke down how the data on patient-reported outcomes is displayed on the website. First, there is a table detailing a large number of symptoms, such as hair loss, nausea, or fatigue, the number of patients treated with the investigational drug and the comparator drug, the percentage of patients with that symptom at baseline, who had worsening of that symptom on treatment, and who had worsening to grade 3 or grade 4 while on treatment.
Patients and providers can than select the symptom of concern, for example, nausea, and click to find out more specifics. For each symptom there is a stacked bar chart showing the frequency with which patients reported that symptom during the first 24 weeks on treatment, broken down on a week-by-week basis. Additionally, there is a pie chart displaying the worst response for that symptom that patients reported during the first 24 weeks on treatment.
Katrina Halling, MSc, of the patient-reported outcomes department at AstraZeneca, and other panelists discussed the importance of balancing the complexity of the data with the simplicity of interpretation.
“This balance will best make information useful to patients and health care providers,” she said.
Experts also discussed how these charts might be improved to help with interpretation, but to also display information on patients who may not have responded to outcomes questionnaires and how that might skew results.
Using the Website
To aid in that understanding, the Project Patient Voice website also includes an Interpretation Guide intended to help further explain the charts used to display data on patient-reported outcomes.
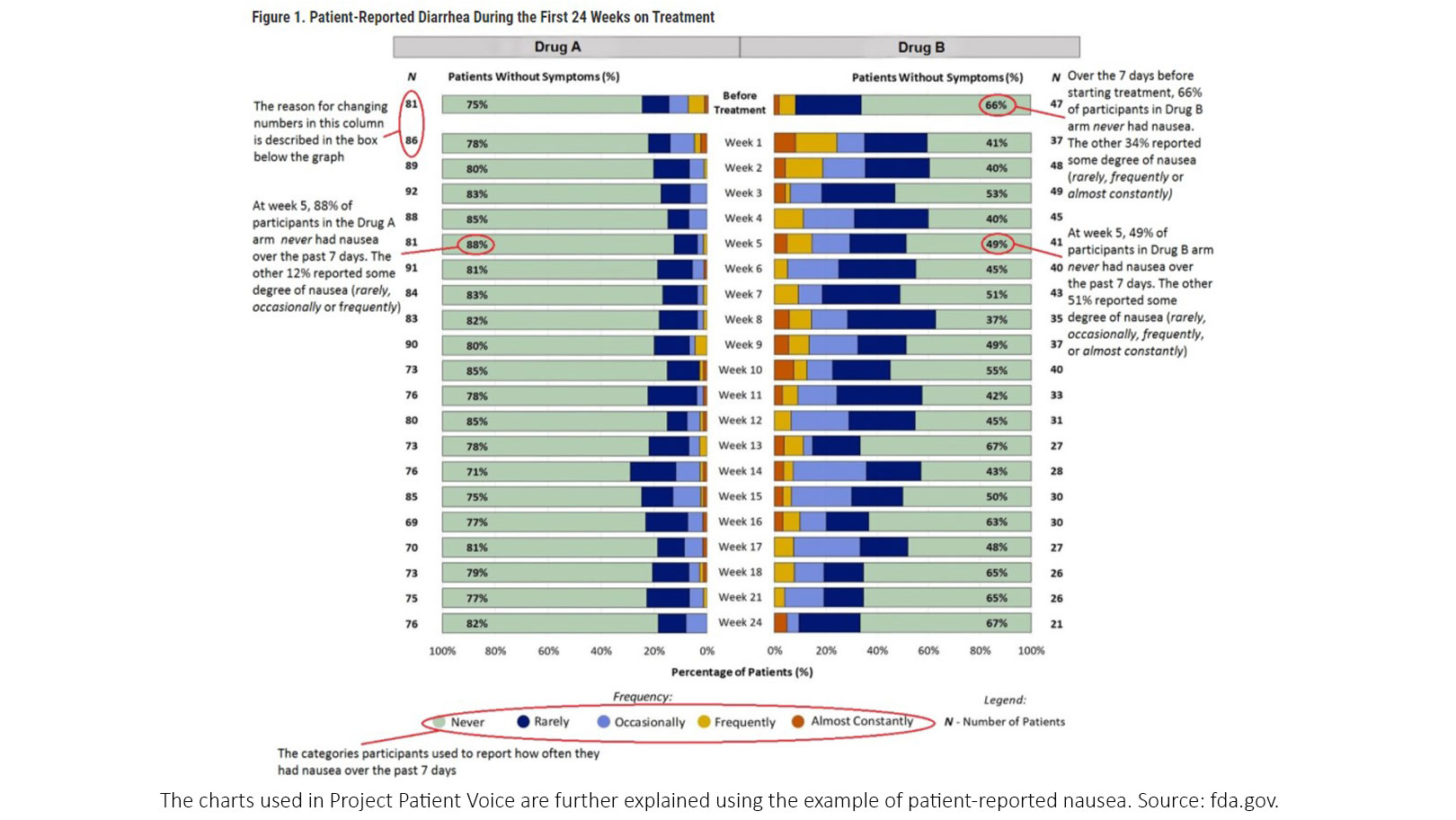
“What we hope to do with this user guide is orient those who are new to the visualization to what things mean,” said Dr. King-Hallimanis. “For example, it might not be immediately clear why the number of patients change each week, but this is because these are snapshots of patients who provided data at each week, so they fluctuate.” (Figure 1)
The workshop also included speakers giving a patient perspective on this new resource. Christine Hodgdon, a patient advocate and breast cancer survivor, said she could envision many patients using this website including those who are considering a new treatment regimen, those with progression who may be looking for a different option, or those who are considering a clinical trial.
“I always get the question, ‘What kind of side effects does this drug have?’” she said. “It is so hard to answer that. Something like this, which is actual data, would be helpful for patients to see.”
Lee Jones, MBA, another patient advocate and colon cancer survivor, said he could envision the website providing even more information, such as details on demographics.
“I think it would be important, as a senior, so I can understand whether this [outcome] would be more or less intense or likely to happen [to me] than for the general population,” Jones said.
He also emphasized that patients should be reminded that the information available on Project Patient Voice should only be one piece of the puzzle for any treatment decision. Patients must also consider efficacy data and data on clinical adverse effects.
Continued Improvement
Other panelists provided the clinicians’ perspective of Project Patient Voice. Karen L. Smith, MD, MPH, of Kimmel Cancer Center at Sibley Memorial Hospital, thought that the tables displaying outcomes may be too busy and wondered if the most clinically important information could be made easier to find.
“Would it be helpful in some way if symptoms for which the largest proportion of patients who worsened to grade 3 or grade 4 were highlighted or bold to show the biggest problems in terms of symptom severity?” Dr. Smith said. She also echoed Jones’ suggestion that demographic information be made available, including possibly a summary about the patient population that completed patient-reported outcomes surveys for each trial.
The FDA welcomes feedback on the use of the Project Patient Voice website and how it might be improved as it continues to add data from new clinical trials.
Providing some closing thoughts on the workshop and Project Patient Voice, Paul Kluetz, MD, of the Oncology Center of Excellence, said one take-home message from the day is that there is no perfect way to visualize data on patient-reported outcomes, but that consistence visualization over time will lead to improved accuracy of interpretation by clinicians who will use this to help guide patients.