
Christine S. Shusted, MPH
Lung cancer is the leading cause of cancer-related mortality in the United States, and besides smoking cessation, annual lung cancer screening (LCS) with low-dose CT (LDCT) is one of the most effective mechanisms for reducing lung cancer mortality.1,2 Moreover, annual LDCT may be even more effective in reducing mortality among Black individuals at risk for lung cancer.2,3 Black individuals tend to have a lower intensity smoking history and present with lung cancer at an earlier age. It is well-established that the U.S. Preventive Services Task Force (USPSTF) LCS eligibility guidelines from 2013 underscreen Black Americans at high risk for lung cancer.4 Furthermore, Black American patients receive disparate lung cancer care; they experience longer intervals between diagnosis and treatment, and are less likely to receive stage-appropriate treatment or early-stage surgical intervention.5,6 Incongruent care and late-stage diagnosis likely contribute to Black Americans experiencing the lowest lung cancer survival rate of any racial group.5
Because patients at high risk of lung cancer derive the greatest benefit from LCS, one approach to mitigate disparities in LCS eligibility is to use lung cancer risk prediction models.7 The PLCOm2012 model8 has been shown to improve sensitivity in lung cancer detection, specifically among Black populations in the United States, and may potentially reduce underrepresentation in screening cohorts.9 Individualized risk-based models may also be more cost-effective and accurate and may decrease the number of high-risk patients currently deemed ineligible.7,10 However, current 6-year lung cancer risk models were validated using the National Lung Screening Trial data, in which only 4.5% of participants were Black Americans.2
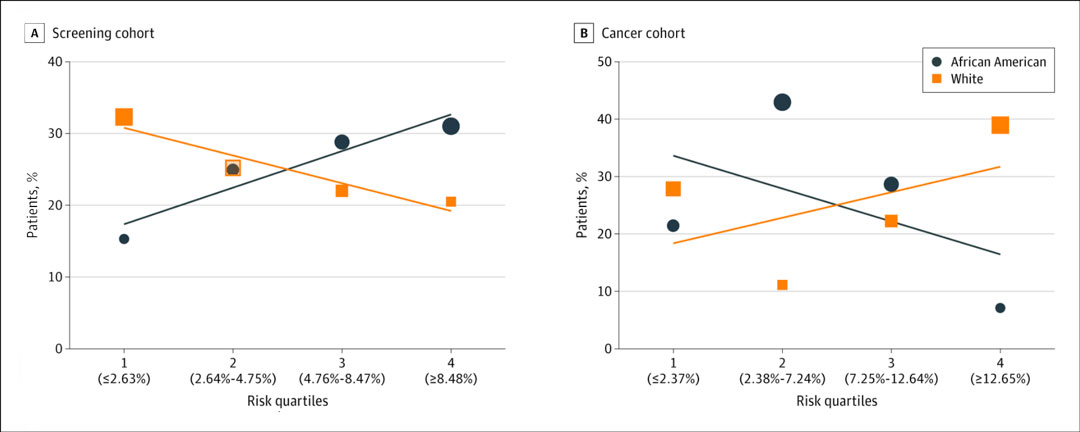
Fig. Six-Year Lung Cancer Risk Among the Screening and Cancer Cohorts by Risk Quartile.
A, Percentage of patients who underwent lung cancer screening in each risk quartile by race. Black and white individuals have overlapping percentages, with 24.9% and 25.2% of each cohort in risk quartile 2.
B, Percentage of patients who had a screen-detected lung cancer in each risk quartile by race. The size of each data icon is proportional to the number of patients in each risk quartile. Risk quartiles are determined by median PLCOm2012 risk values (with cutoffs shown in parentheses along the x-axis).
Reprinted from Shusted CS, Evans NR, Juon H-S, Kane GC, Barta JA. Association of Race With Lung Cancer Risk Among Adults Undergoing Lung Cancer Screening. JAMA Netw Open. 2021;4(4):e214509.
A recent study at Thomas Jefferson University in Philadelphia aimed to investigate race-dependent differences in 6-year lung cancer risk among patients in a centralized LCS program at an urban, academic, medical center.11 During a 33-month period, 1,276 patients who self-identified as Black or white were screened through Thomas Jefferson University’s LCS program. The program consists of an eligibility assessment upon referral, conducted by the program coordinator; a shared decision-making session with a nurse navigator; extensive results review; and concierge scheduling of follow-up tests, procedures, and appointments, if applicable.
For each patient enrolled in the program, medical, family, and smoking history as well as demographic and socioeconomic data are collected via a standardized intake form and entered directly into the program’s registry. On the basis of the data collected during intake, each patient’s 6-year lung cancer risk is systematically calculated using the validated PLCOm2012 model.8
Of the 1,276 patients screened, 128 received a positive scan result (Lung-RADS 3, 4A, 4B, or 4X), and 32 were diagnosed with screen-detected lung cancer. Risk quartiles were generated using the median PLCOm2012 risk scores for the screening and cancer cohorts. Descriptive statistics, independent t-tests, chi square tests, and Mann–Whitney U tests were performed using a significance threshold of p < 0.05 to ascertain racial differences in PLCOm2012 scores.

Julie A. Barta, MD, ATSF
In the screening cohort, 42.7%, or 545 of 1,276 patients, were Black. In the cancer cohort, 43.8%, or 14 of 32 patients, were Black. There were significant differences in gender distribution within the screening cohort: 62.9%, or 343, of the Black patients were female, compared with 56.6%, or 414, of the white patients (p = 0.02). Current smokers included 348 Black patients and 358 white patients, comprising a significantly different percentage of the two groups, 63.9% and 49.0% respectively (p < 0.001). The distribution of educational attainment differed significantly between Black and white patients (p < 0.001). Within the cancer cohort, adenocarcinoma was the most frequent lung cancer histology, with 78.6% of Black and 55.6% of white individuals having this subtype. Of all individuals with screen-detected lung cancer, 66% were diagnosed with stage I or II NSCLC or limited-stage SCLC.
Black patients in the screening cohort had a significantly higher median 6-year lung cancer risk score compared to white patients, with greater distribution of Black patients in higher risk quartiles (60% of African American vs. 43% of white patients in Quartile 3 and Quartile 4; Fig.). In contrast, in the cancer cohort, Black patients had a lower median PLCOm2012 risk, with greater distribution in lower risk quartiles (64% of Black patients vs. 39% of white patients in Quartile 1 and Quartile 2). These data suggest that lung cancer risk scores were not aligned with lung cancer diagnoses in Black patients.
Many existing risk models have been developed and validated in clinical trials that have included a small proportion of individuals of minority race, and these models focus on traditional lung cancer risk factors that have been largely identified among white patients.2,7 However, we know that social determinants and other sociocontextual, cultural, and environmental factors may be critical moderators of lung cancer risk among vulnerable populations.12,13 Notably, our screening cohort has a higher median lung cancer risk overall compared with other reported populations.8,9
In addition to having the highest adult smoking rate among large cities, Philadelphia is the poorest large city in the United States and has the third-largest income gap in the country, one that persists across all races and ethnicities but is more significant for communities of color.14 The city consistently ranks worse than the national average on several known lung cancer risk factors, including education, obesity, and poverty.14 Additional research on comprehensive lung cancer risk prediction for vulnerable populations should focus on potentially critical confounders related to socioeconomic status, geographic location, the environment, and exposure history.
Recently updated USPSTF LCS guidelines have expanded lung cancer screening criteria on the basis of age and smoking history, thus increasing the number of eligible individuals.15 It is expected that the new USPSTF recommendation will lead to an increase in screening, specifically among women and Black and Latino individuals.16 Although this is an encouraging first step, the focus needs to remain on developing strategies to overcome barriers that perpetuate lung cancer screening disparities.17
Clinicians, public health professionals, and communities must come together to dismantle long-held perceptions and attitudes around lung cancer screening, lung cancer, and healthcare as a whole and to reframe preventive LDCT as a critical piece of overall annual care for those who are at high risk. Further, barriers must be addressed and overcome not only at an individual level but also throughout all levels of the socioecological model in order to create an equitable landscape in both lung cancer screening and lung cancer–related outcomes.
References:
- 1. Ru Zhao Y, Xie X, de Koning HJ, Mali WP, Vliegenthart R, Oudkerk M. NELSON lung cancer screening study. Cancer Imaging. 2011;11 Spec No A(1A):S79-S84.
- 2. National Lung Screening Trial Research T, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
- 3. Tanner NT, Gebregziabher M, Hughes Halbert C, Payne E, Egede LE, Silvestri GA. Racial Differences in Outcomes within the National Lung Screening Trial. Implications for Widespread Implementation. Am J Respir Crit Care Med. 2015;192(2):200-208.
- 4. Aldrich MC, Mercaldo SF, Sandler KL, Blot WJ, Grogan EL, Blume JD. Evaluation of USPSTF Lung Cancer Screening Guidelines Among African American Adult Smokers. JAMA Oncol. 2019;5(9):1318-1324.
- 5. Lin JJ, Mhango G, Wall MM, et al. Cultural factors associated with racial disparities in lung cancer care. Ann Am Thorac Soc. 2014;11(4):489-495.
- 6. Wolf A, Alpert N, Tran BV, Liu B, Flores R, Taioli E. Persistence of racial disparities in early-stage lung cancer treatment. J Thorac Cardiovasc Surg. 2019;157(4):1670-1679.e4.
- 7. Katki HA, Kovalchik SA, Berg CD, Cheung LC, Chaturvedi AK. Development and Validation of Risk Models to Select Ever-Smokers for CT Lung Cancer Screening. JAMA. 2016;315(21):2300-2311.
- 8. Tammemagi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening. N Engl J Med. 2013;368(8):728-736.
- 9. Pasquinelli MM, Tammemägi MC, Kovitz KL, et al. Risk Prediction Model Versus United States Preventive Services Task Force Lung Cancer Screening Eligibility Criteria: Reducing Race Disparities. J Thorac Oncol. 2020;15(11):1738-1747.
- 10. Meza R, Jeon J, Toumazis I, et al. Evaluation of the Benefits and Harms of Lung Cancer Screening With Low-Dose Computed Tomography: Modeling Study for the US Preventive Services Task Force. JAMA. 2021;325(10):988-997.
- 11. Shusted CS, Evans NR, Juon H-S, Kane GC, Barta JA. Association of Race With Lung Cancer Risk Among Adults Undergoing Lung Cancer Screening. JAMA Netw Open. 2021;4(4):e214509.
- 12. Haddad DN, Sandler KL, Henderson LM, Rivera MP, Aldrich MC. Disparities in Lung Cancer Screening: A Review. Ann Am Thorac Soc. 2020;17(4):399-405.
- 13. Sosa E, D’Souza G, Akhtar A, et al. Racial and socioeconomic disparities in lung cancer screening in the United States: A systematic review. CA Cancer J Clin. 2021;71(4):299-314.
- 14. Philadelphia Department of Public Health. 2017 Community Health Assessment (CHA) Philadelphia PA. Philadelphia, PA: Philadelphia Department of Public Health;2017.
- 15. United States Preventive Services Task Force. Final Recommendation Statement: Lung Cancer: Screening. March 9, 2021. Accessed August 10, 2021. https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/lung-cancer-screening
- 16. Reese TJ, Schlechter CR, Potter LN, et al. Evaluation of Revised US Preventive Services Task Force Lung Cancer Screening Guideline Among Women and Racial/Ethnic Minority Populations. JAMA Network Open. 2021;4(1):e2033769.
- 17. Rivera MP, Katki HA, Tanner NT, et al. Addressing Disparities in Lung Cancer Screening Eligibility and Healthcare Access. An Official American Thoracic Society Statement. Am J Respir Crit Care Med. 2020;202(7):e95-e112.




