While several phase II studies have demonstrated that local consolidation therapy (LCT) can improve event-free survival outcomes compared to systemic therapy alone, none have specifically evaluated primary tumor resection (PTR) in combination with EGFR TKI therapy before disease progression.
Researchers evaluated whether PTR as LCT in metastatic non-small cell lung cancer (NSCLC) could improve outcomes beyond those achieved with EGFR TKI monotherapy. The results from the randomized phase II PTR-1 trial were presented by Pei-Hsing Chen, MD, in September at the 2025 World Conference on Lung Cancer (WCLC) in Barcelona.

Expanding Horizons in Advanced NSCLC Integrating Local Therapy
Registered WCLC 2025 attendees can watch Dr. Pei-Hsing Chen’s presentation, Molecular and Early Outcomes of a Randomized Phase II Trial of Primary Tumor Resection Following EGFR TKI in Metastatic NSCLC, and other WCLC content on demand. Learn More
The two-arm, phase II PTR-1 trial enrolled 91 patients with EGFR-mutation (+) advanced NSCLC, including both oligometastatic and polymetastatic populations. Dr. Chen said the trial is the first of its kind to evaluate primary tumor resection specifically as local consolidation therapy alongside EGFR TKI in both oligometastatic and polymetastatic NSCLC.

Nineteen participants were excluded for either not meeting eligibility requirements or declining to participate. Following 12 weeks of afatinib, 72 participants were randomized (1:1) to either continue afatinib monotherapy or to afatinib and surgery to remove the primary tumor. Radiotherapy was allowed for non-pulmonary lesions. The majority of participants were women and individuals with no previous tobacco smoking history.
Dr. Chen said all patients on the surgery arm underwent minimally invasive resections following EGFR TKI treatment, with few surgical complications and no surgery-related mortality. The median time from drug to surgery was 3.7 months in the TKI-plus-PTR arm.
The primary endpoint was 2-year progression-free survival (PFS); secondary endpoints included overall PFS and overall survival (OS). Postoperative specimens were assessed for pathological response and molecular profiling in patients with sufficient residual tumor tissue.
The PFS hazard ratio (HR) was 0.48, though data remain immature, Dr. Chen said. Major pathological response (MPR) was observed in 29.4% of the PTR arm, and pathological complete response (pCR) was observed in 5.9%.
In the TKI-plus-PTR group, exon 19 deletion was associated with a higher pathological response rate in the primary tumor than L858R. However, the L858R subgroup showed a lower HR for PFS (0.38) than the exon 19 deletion subgroup (0.60).
Major pathological response has not yet correlated with improved survival, he added.
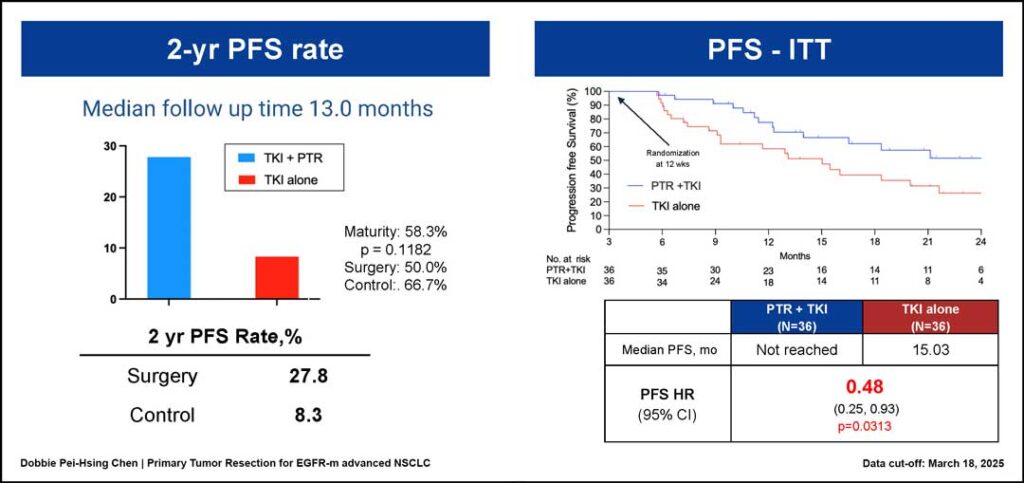
After the median follow-up of 13 months, the 2-year PFS rate was 27.8% for the TKI-plus-PTR arm and 8.3% for the TKI-alone arm. The median PFS was 15.03 months for the TKI-alone arm, but it had not yet been reached for the TKI-plus-PTR arm (hazard ratio [HR] 0.48; 95% confidence interval [CI]: 0.25–0.93; p = 0.0313).
Median PFS was not reached in the oligometastatic population in the PTR-plus-TKI arm; however, it was 15.47 months in the TKI-only group. Among those in the polymetastatic population, the median PFS was 18.37 months in the PTR-plus-TKI group versus 9.1 months in the TKI-only arm.
Further, Dr. Chen said the data showed a numerical PFS benefit for those with exon 19 deletion in the TKI-plus-PTR group compared with the TKI-only arm (21.13 months vs. 16.03 months). Median PFS was not reached for those with L858R mutations in the TKI-plus-PTR group compared to 9.1 months for those in the TKI-only arm.
Dr. Chen said the promising early PFS results suggest that removing the primary lung tumor following EGFR TKI treatment may be a safe and effective approach to prolong disease control in patients with advanced NSCLC.
This approach offers new insights for future subgroup resection by providing access to postoperative pathological and molecular data, he said, adding that the evidence suggests such a strategy may be especially beneficial for patients with L858R mutations, though additional follow-up is needed to understand long-term outcomes.
“Primary tumor resection is not a cure in stage IV lung cancer, but it still provided a possible combination, just like in the FLAURA2 and MARIPOSA setting,” Dr. Chen said. “We might provide better disease control compared with mono EGFR TKI treatments, especially for those with the worst treatment response on systemic treatment, such as the L858R subgroup.”










