In the following Q&A, Luis E. Raez, MD, explains his therapeutic decision making process and why he favors amivantamab plus lazertinib as the new standard of care for first-line treatment of patients with locally advanced or metastatic NSCLC with EGFR exon 19 deletions or L858R substitution mutations. Dr. Raez also provides insights about the role of chemotherapy in the overall treatment plan and optimal patient selection. In addition, Beth Sandy, MSN, CRNP, discusses best practices for incorporating novel data into shared decision making with patients, as well as setting accurate expectations regarding adverse events.
Luis E. Raez, MD, works as Chief Scientific Officer & Medical Director at Memorial Cancer Institute (MCI)/Memorial Health Care System, where he is also Director of the Thoracic Oncology Program. He is Co-Director of the MCI/FAU Florida Cancer Center of Excellence designated by the Florida Department of Health, one of only five cancer centers in Florida. He is also Clinical Professor of Medicine at Florida International University (FIU), Visiting Professor of Medicine at Cayetano Heredia University in Peru, and Research Professor at Florida Atlantic University (FAU).
What strikes you as important regarding the MARIPOSA data presented at European Lung Cancer Congress 2025 (ELCC) on overall survival rates for amivantamab plus lazertinib compared with osimertinib alone in the first-line setting?

In previously reported MARIPOSA data, there was a progression-free survival (PFS) benefit of approximately 30% for the combination (HR, 0.70; p < 0.001).1,2 Although this information alone was enough to garner approval in the United States for this combination as first-line therapy for these patients, there was controversy about whether PFS is enough to change the standard of care. The current standard of care for first line is osimertinib plus chemotherapy, which is based on FLAURA2 results.3 So we have spent the past year or two debating, with some physicians adopting the MARIPOSA regimen and some staying with the FLAURA2 regimen, until we had consistent data looking at overall survival (OS) to use for the basis of therapeutic decision making.
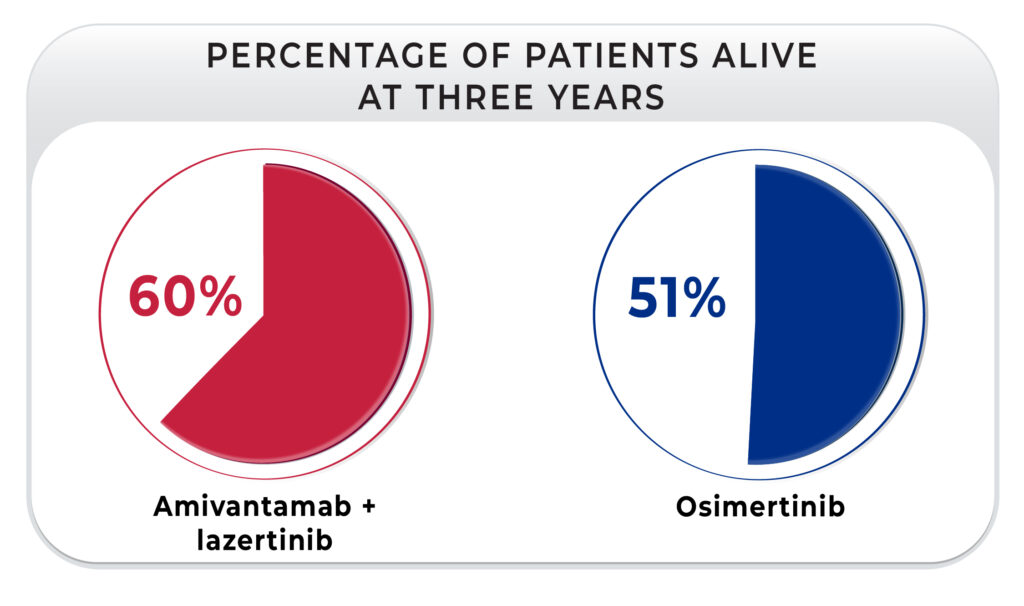
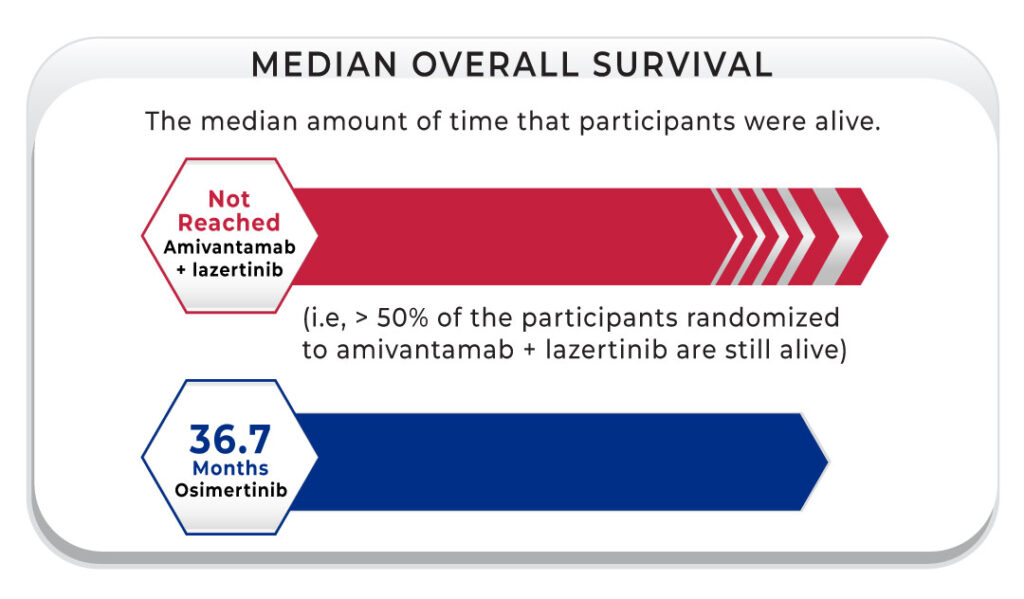
This brings us to why the presentation at the ELCC in March 2025 was very important. First, the hazard ratio for the OS was 0.75, so there is a 25% benefit in survival with the combination of amivantamab plus lazertinib.4,5 In addition, 60% of patients who received the combination were alive at three years compared with 51% of those who received osimertinib, with a continued benefit shown at 42 months (56% vs. 44%, respectively). It is very interesting that the median OS for the osimertinib group was 36 months, but the group that received amivantamab plus lazertinib has not reached median survival yet. These should be the data that those physicians who were hesitant to switch in the first line from osimertinib plus chemotherapy to amivantamab plus lazertinib look to now.
How does this factor into your overall therapeutic decision making?
I think that there should not be any doubt that combination therapy (MARIPOSA or FLAURA2) is the new standard of care and we should no longer use osimertinib alone unless we have an exception. For example, in Florida we have a large senior population. I have five patients older than 90 and more in their 80s so maybe osimertinib alone would be better for this population, as well as for those with a performance status of 2 or those with comorbidities, based on toxicity. But the standard of care is the combination amivantamab plus lazertinib or the FLAURA2 combination; that must be clear.
Despite this, it remains to be seen what strategy physicians are going to prefer. I think it is possible that the FLAURA2 regimen is very popular because physicians feel very comfortable with chemotherapy agents like carboplatin and pemetrexed, which we have been using for 20 years or more. So, we are very familiar with the side effects from chemotherapy. But the survival data for MARIPOSA are just very appealing. In the past, we used to approve lung cancer drugs based on a two-month survival benefit; now we have drugs that achieve more than one year of benefit. I think the efficacy data are very important.
But there are other reasons I prefer the MARIPOSA combination. For example, one of the mechanisms of resistance found with EGFR inhibitors is MET amplification, which is lower than with osimertinib. In addition, amivantamab plus lazertinib goes to the brain, with a three-year landmark intracranial PFS rate of 36% for the combination and just 18% for osimertinib alone.4 This was big news, as amivantamab is a large molecule so we did not expect to see intracranial activity.
Remember lung cancer does not die easily so we need to use multiyear therapies. One of the benefits of osimertinib plus chemotherapy in the first line is that you can give pemetrexed for a long time, but in FLAURA2 pemetrexed was only given up to seven cycles.3 So, in other words, you put a patient on the FLAURA2 regimen in the first line and one year later that patient is on osimertinib alone. With amivantamab plus lazertinib in the first line, we have the benefit of knowing that one year later that patient can still be on the same combination. MARIPOSA data showed us that the time to symptomatic progression for the combination was 43.6 months versus 29.3 months for osimertinib alone (HR, 0.69; 95% CI, 0.57-0.83, p < 0.001).4,5 We are not erasing the possibility of using chemotherapy by using this combination in the first line. If the combination fails, of course you could use chemotherapy first, and you can also use amivantamab alone after that. But we are also back to the point I made earlier about efficacy, in that you always want to use the most efficacious treatment first because, historically, more than one-third of patients do not get second-line therapy.
When it comes to combination therapies, how do you balance efficacy with side effect management?
Lung cancer is very lethal. Even today, we lose approximately 70% to 75% of patients across all disease stages in the first five years from diagnosis.6 So it’s not like we have the luxury of looking for a therapy that can prioritize quality of life as the main factor. Without therapy, there is no life. I think this is a very important point. This is not prostate cancer, where sometimes a physician can watchfully wait for two years before starting therapy. In lung cancer, efficacy is the most important thing because this is a very lethal disease that leads to dramatic shortening of a patient’s life span. Of course we want to preserve quality of life, and I think the management of side effects is crucial to this.

[A]mivantamab plus lazertinib goes to the brain, with a three-year landmark intracranial PFS rate of 36% for the combination and just 18% for osimertinib alone.
Luis E. Raez, MD
With any cancer drug that gets approved, the ability to successfully manage the drug’s associated side effects improves tremendously the more it is used in clinical practice. There are a lot of drugs like oxaliplatin, cetuximab, and even paclitaxel that originally seemed to be very toxic agents. We used to keep patients sitting in the chair after infusion for hours for observation of adverse events, but now all of us know how to deal with these agents so our approach has evolved. The more that physicians use a new therapy, like combination amivantamab and lazertinib, the more we learn how to deal with adverse events, so they do not overwhelm us or our patients. For example, grade 3 rash is very bothersome because it covers more than 50% of the body, so we need to know how to prevent and manage it. But we also need to remember that this will occur for maybe one patient out of every five. Also, the adverse events get better with time. The first three or four months are the toughest months for the rash and the infusion reactions, but they get much better, much easier. And then you have a maintenance therapy a patient can be on for a long time.
Going forward, how will you discuss all first-line regimens with your patients?
I will outline the two different combinations and let them know that I would like to focus on efficacy. Obviously, I will inform them about the safety data associated with each combination, and then I will let the patient have some time to decide. I do not like it when the physician tells the patient that they are choosing a therapy because it has good quality of life because that scenario is just rushed. The decision about quality of life is personal. I do not think it is fair for physicians to assume what a patient can or cannot handle. I think we must take the time to discuss each patient’s personal view of quality of life and empower the patient through education about side effects and their management, including prophylactic and reactive strategies. I think empowering the patient with information so that they can have a choice is very important. If you do not provide any information because you have decided what “quality of life” means and the patient gets a terrible rash at week 3, he is going to be scared and uncertain.
I worked with several colleagues to put together an update for the ASCO/NCODA Patient-Centered Standards for Medically Integrated Dispensing, originally published in 2019. We will be publishing the updated version in the Journal of Clinical Oncology Practice, which will provide a lot of information and best practices for specialty pharmacies and physicians to better empower patients about safety and adverse events. Pharmacists or advanced practice providers at my institution will spend an hour or so teaching patients about side effects of drugs as they move into daily clinical care, which is very helpful. These updated standards do the same in that they go beyond what you can read on the drug’s website. Specific to amivantamab plus lazertinib, it is important to remember that prophylactic anticoagulation substantially reduces the incidence of venous thromboembolism,7 prophylactic steroids result in a three-fold decrease in infusion reactions,8 and when we use antibiotics and other treatments for skin and nails prophylactically, subsequent rash can be reduced by as much as 70%.9
Beth Sandy, MSN, CRNP, is a nurse practitioner at the Abramson Cancer Center at the University of Pennsylvania specializing in lung and head and neck cancers. Ms. Sandy holds various editorial positions for oncology journals and newsletters, and she is a current Board member of the Advanced Practitioner Society for Hematology and Oncology (APSHO).
Newly diagnosed patients can suffer information overload. How do you navigate shared decision-making conversations with this in mind, especially when a prolonged time to treatment may affect overall outcomes?

As the first visit is with the physician, I do get a lot of questions from patients at the second visit, which is usually when treatment is initiated. Although treatment has been selected prior to this visit with me, we discuss potential side effects at a granular level including the potential intensity and duration of side effects. Although I have had patients change their minds about treatment at this visit due to a better understanding about potential side effects, the vast majority of patients want to take the treatment that is going to give them the best outcome at the end of the day. Therefore, I think it is really incumbent on us, the advanced practice providers who are spending the most time with the patients, to do our best to maximize supportive care strategies related to each therapy.
So for example, oftentimes I will talk about rash with patients who will be receiving an EGFR inhibitor. I let them know that the rash is likely to occur within the first week or two and then it may wax or wane over time. I set expectations for them about appearance by letting them know that the rash may look like teenage acne and it is often, unfortunately, on their face and chest. I think it is very important to pause and give the patient time to think about that. The majority of my patients are scared of the cancer itself; they want to get it better, and if this is the best treatment, that’s what they’re going to do no matter what. I did have one patient who was a hairdresser, and she did not love that her appearance could potentially be affected. So we talked a lot about how to mitigate rash while still feeling confident in her appearance, such as using moisturizer and makeup made for sensitive skin. I had another patient who was a trial attorney and was worried that a rash on his face could be detrimental to his career when facing a jury for arguments, so we were very aggressive with trying to proactively minimize his rash.

Although I have had patients change their minds about treatment at this visit due to a better understanding about potential side effects, the vast majority of patients want to take the treatment that is going to give them the best outcome at the end of the day.
Beth Sandy, MSN, CRNP
I also provide the patient with printed educational materials from OncoLink®, which we use at my institution and find to be a really good resource for patient education. I always stress that every single side effect anyone has ever experienced is often listed on the educational materials, but that does not mean each patient is going to experience all effects. I carry a highlighter in my pocket, and I usually will highlight the most common side effects, what the patient is more likely to experience. And then I like to assign a timeline to each common side effect as well, which I write next to it on their handout.
At these visits, especially where there is toxicity management involved, the main caregiver is strongly encouraged to attend and participate in the discussion. Not only is that a second set of ears to remind the patient of what was said, but it is also the person who is likely to observe how a treatment affects the patient in daily life. The patient is not always necessarily going to tell us about changes in quality of life, but the spouse often does, let me tell you. I try really hard in my visits to include the caregiver through eye contact and questions, because I want both the patient and the caregiver to understand that both of them are really important in this journey and that I will be looking for answers to my questions from both of them.
What are some best-practice recommendations for discussing novel trial data with newly diagnosed patients?
Everything is new to a patient when they are newly diagnosed with lung cancer. So whether it is new data that we as providers get excited about or older data that has found its place within the standard of care, I find that in my practice, even at a large academic institution, the majority of patients are not super fixated on data. They typically want a high-level overview; it is uncommon that patients ask for specific PFS or OS numbers related to a specific treatment regimen. And even if they do say, “What is the average survival for this treatment?” I will say, “Do you want actual numbers or do you want the big picture?” Because I have found that patients can get very fixated on a number, such as median OS. Once patients fixate on a number, all they do is count down. I do not want patients to feel like they are counting down their lives so I always remind them everyone is very different. I will tell them that, “An average means that half the patients do better and half the patients do worse than that number. So we will need to watch for response on scans and see how you feel as we move further into treatment to determine where you are falling in relation to the average.”
How will you use the MARIPOSA data reported at ELCC 2025 specifically in shared decision-making conversations?
I think when we are talking about newly diagnosed EGFR mutation-positive lung cancer, we are going to have a couple different options for them now, all of which are going to include an oral EGFR inhibitor that can have some toxicity. And then the question is whether they are going to want to add chemotherapy to that or add another intravenous EGFR antibody like in the Mariposa trial, which has shown some very impressive OS data in the most recent ELCC data.4,5 And so this is something I think that probably we are going to want to offer patients.

Again, whether it is related to logistics or potential adverse events, there will be more to discuss with patients regarding the MARIPOSA regimen but, generally, patients in my practice have always been more motivated by survival.
Beth Sandy, MSN, CRNP
Advanced practitioners should definitely talk with patients and caregivers about logistics as early as possible because amivantamab is infused weekly initially and then it moves to every-other week. If chemotherapy is used instead, that would be once every three weeks, so the logistics are a bit more convenient. Obviously we know that there may be infusion-related reactions commonly seen with the MARIPOSA regimen, but we also know how to reduce those prophylactically using 8 mg of oral dexamethasone given twice daily two days before and again one day before, as well as on the morning of the first infusion, as shown in the SKIPPirr trial data.8 Again, whether it is related to logistics or potential adverse events, there will be more to discuss with patients regarding the MARIPOSA regimen but, generally, patients in my practice have always been more motivated by survival.
References
- 1. Cho BC, Lu S, Felip E, et al. Amivantamab plus Lazertinib in Previously Untreated EGFR-Mutated Advanced NSCLC. N Engl J Med. 2024;391:1486-1498.
- 2. Cho BC, Felip E, Spira AI, et al. LBA14 – Amivantamab plus lazertinib vs osimertinib as first-line treatment in patients with EGFR-mutated, advanced non-small cell lung cancer (NSCLC): Primary results from MARIPOSA, a phase III, global, randomized, controlled trial. Presented at the European Society for Medical Oncology (ESMO) Congress; October 20-24, 2023.
- 3. Planchard D, Jänne PA, Cheng Y, et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N Engl J Med. 2023;389:1935-1948.
- 4. Yang JC-H, Kim YJ, L S-H, et al. Amivantamab Plus Lazertinib vs Osimertinib in First-line EGFR-mutant Advanced NSCLC: Final Overall Survival from the Phase 3 MARIPOSA Study. Presented at European Lung Cancer Congress 2025; March 26-29, 2025.
- 5. Yang JC-H, Kim YJ, L S-H, et al. 40: Amivantamab plus lazertinib vs osimertinib in first-line (1L) EGFR-mutant (EGFRm) advanced NSCLC: Final overall survival (OS) from the phase III MARIPOSA study. J Thorac Oncol. 2025;20(3_Suppl 1):S6-S8.
- 6. American Cancer Society. Lung Cancer Survival Rates. Published online January 29, 2024. Accessed April 8, 2025. https://www.cancer.org/cancer/types/lung-cancer/detection-diagnosis-staging/survival-rates.html
- 7. Besse B, Goto K, Wang Y, et al. Amivantamab Plus Lazertinib in Patients With EGFR-Mutant NSCLC After Progression on Osimertinib and Platinum-Based Chemotherapy: Results From CHRYSALIS-2 Cohort A. J Thorac Oncol. Published online January 1, 2025. DOI: 10.1016/j.jtho.2024.12.029.
- 8. Spira AI, Paz-Ares L, Han J-Y, et al. Preventing Infusion-Related Reactions With Intravenous Amivantamab—Results From SKIPPirr, a Phase 2 Study: A Brief Report. J Thoracic Oncol. Articles in press. Published online January 24, 2025.
- 9. Girard N, Li W, Spira AI, et al. Preventing Moderate to Severe Dermatologic Adverse Events in First-line EGFR-Mutant Advanced NSCLC Treated with Amivantamab Plus Lazertinib: Early Success of the COCOON Trial. Presented at European Lung Cancer Congress 2025; March 26-29, 2025.