Editor’s Note: In Part 2 of our series on the NRG-CC003 trials, Drs. Tomasik and Dziadziuszko continue a conversation regarding prophylactic brain irradiation and stereotactic brain radiotherapy for metastases. Part 1, Modern Brain Radiotherapy in SCLC: Integrating Hippocampal Avoidance and Stereotactic Precision, is available now.

Early brain metastases are common in small cell lung cancer (SCLC), which historically was managed with upfront whole-brain radiotherapy (WBRT) even for patients with limited central nervous system (CNS) involvement. However, recent evidence suggests that carefully selected patients with SCLC limited brain metastases can safely receive stereotactic radiosurgery (SRS) with close MRI surveillance. The multi-institutional FIRE-SCLC cohort (Rusthoven et al., 2020) analyzed 710 patients with SCLC who were treated initially with SRS (no prior PCI/WBRT).1
Median overall survival (OS) with SRS alone was ~8-9 months for all patients and 11 months in those with a single metastasis, comparable to historical outcomes with WBRT. While intracranial recurrences were common (12-month distant brain failure ~42%), the rate of neurological death remained low (~12%). Notably, in a propensity-matched comparison, upfront WBRT prolonged time to CNS progression, but did not improve OS relative to SRS (median OS ~6.5 vs. 5.2 months).
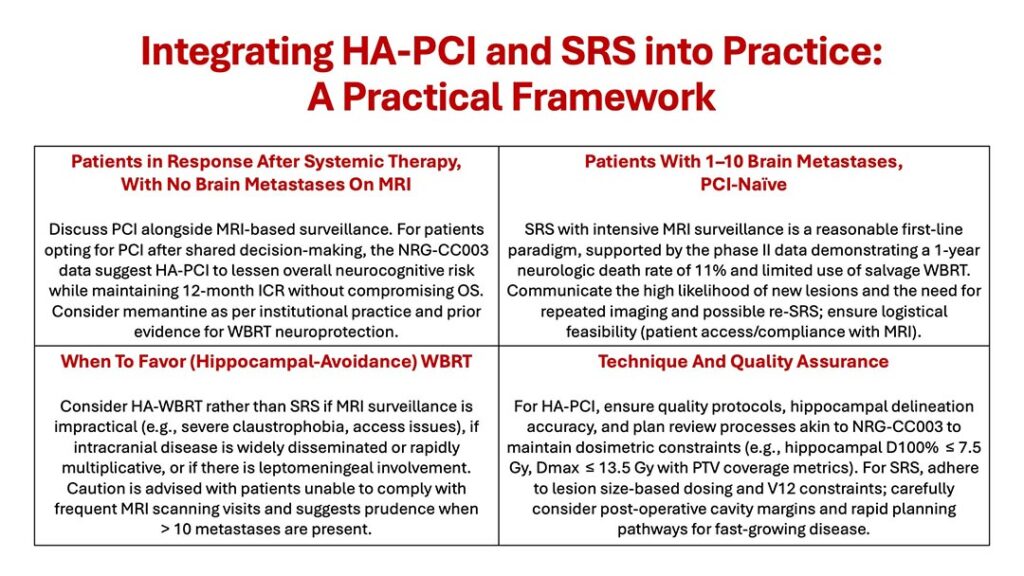
These retrospective results have been reinforced by prospective data. A multicenter, single-arm, phase II trial by Aizer et al. enrolled 100 patients with SCLC (or extrathoracic small-cell primaries) and 1-10 brain metastases, naïve to prior brain-directed radiotherapy (including PCI). The trial’s primary objective was to determine whether SRS/SRT could limit neurologic death to acceptable levels relative to a historical WBRT cohort. Crucially, the protocol mandated frequent MRI surveillance: imaging 4 weeks after SRS, 6 weeks thereafter, and then every 8 weeks until 1 year, with additional scans as clinically indicated. Dose/fractionation reflected contemporary practice (e.g., 20 Gy × 1 for < 2 cm lesions, or multi-fraction regimens to respect V12Gy and OAR constraints).
At one year, the neurologic death rate was 11% (95% CI 5.8-18.1), lower than the historical WBRT benchmark (17.5%) cited by the investigators, derived from an institutional series of patients treated between 2008 and 2015, when SRS/SRT was not used. Salvage WBRT was required in only 22% of patients; median OS was 10.2 months. These data provide the first prospective, multi-institutional dataset demonstrating that SRS can be used upfront in patients with SCLC and a limited number of brain metastases, provided vigilant MRI surveillance is followed.
These results underscore a practical reality: despite acceptable neurologic death rates, new brain metastases occurred in 61% of patients, necessitating further intracranial therapy in many cases (e.g., additional SRS in 39% or WBRT in 22%). Such patterns underscore the observation that an SRS-first strategy can de-escalate initial brain irradiation but does not eliminate the need for close monitoring and potential retreatment. Additionally, starting with SRS preserves WBRT as a salvage option should diffuse failure or leptomeningeal disease subsequently emerge.
Unresolved Questions and Future Directions

The key gap in evidence remains the comparative effectiveness of SRS versus HA-WBRT for patients with SCLC with a limited number of brain metastases. The ongoing NRG-CC009 randomized trial (NCT04804644) is designed to address this question prospectively, randomly assigning patients with SCLC and up to 10 metastases to SRS versus HA-WBRT; timely enrollment is encouraged given the high prevalence and clinical impact of SCLC brain disease. Until those data mature, clinicians must individualize care using the CC003 and Aizer et al. data, layered on patient preferences, shared decision-making, and institutional recommendations.
Other areas also merit attention. First, systemic therapy integration, particularly with chemo-immunotherapy regimens common in extensive-stage SCLC, may influence CNS control and neurotoxicity profiles, but high-quality prospective data specific to brain-directed radiotherapy choices remain sparse.
Exploratory analysis of the ADRIATIC trial presented during the European Lung Cancer Congress 2025 indicated that durvalumab consolidation in limited-stage SCLC reduced the rate of extrathoracic metastases and prolonged time to progression or death for patients with intrathoracic and extrathoracic metastases, including brain and central metastases, and that durvalumab reduced the risk of brain and CNS metastases regardless of PCI use.2,3
Notably, patients receiving PCI in both arms had better outcomes than those who did not. In addition, novel bispecific antibodies targeting the DLL3 protein, expressed almost universally in SCLC, such as taralatamab, obrixtamig, and gocatamig, are very likely to affect the discussion of optimal brain control from the systemic side of the equation. Another important trial is PRIMALung (EORTC-1901) comparing PCI (hippocampal avoidance techniques are permitted, but not mandatory) with active brain MRI surveillance in patients with SCLC, aiming for non-inferior survival and improved cognition in the era of immunotherapy.4
In addition, the phase III MAVERICK trial (SWOG S1827) is also comparing regular MRI surveillance alone to PCI (with MRI) in patients with SCLC after initial therapy (including immunotherapy), to determine if observation is non-inferior with respect to OS. This study also allows modern techniques (e.g., HA-PCI) and salvage SRS/WBRT as needed.
Second, biomarkers of neurocognitive susceptibility and of CNS metastatic propensity could refine selection for HA-PCI versus surveillance or SRS versus HA-WBRT. Finally, pragmatic research on surveillance intensity, patient-reported outcomes, and health-system logistics (MRI access, rapid salvage capacity) will determine the real-world success of brain-sparing strategies.
Conclusion
The SCLC field is moving toward brain-sparing radiotherapy to maximize disease control as well as spare cognitive damage. In the prophylactic setting, HA-PCI appears to reduce overall neurocognitive toxicity without sacrificing intracranial control or survival among patients who choose PCI. In the therapeutic setting for limited brain metastases, SRS with intensive surveillance offers a feasible alternative to immediate WBRT, with promising neurologic outcomes and the advantage of preserving WBRT as a future option.
These strategies are not mutually exclusive but rather complementary tools for tailoring care. As we await the results of NRG-CC009, practices should emphasize shared decision-making, transparent counseling about surveillance commitments, and institutional readiness for rapid, iterative salvage—principles that align the oncologic goals of control and survival with the human priority of cognitive preservation.
References
- 1. Rusthoven CG, Yamamoto M, Bernhardt D, Smith DE, Gao D, Serizawa T, et al. Evaluation of First-line Radiosurgery vs Whole-Brain Radiotherapy for Small Cell Lung Cancer Brain Metastases: The FIRE-SCLC Cohort Study. JAMA Oncol. 2020 Jul 1;6(7):1028–37.
- 2. Senan S, Cheng Y, Spigel DR, Cho BC, Laktionov K, Zenke Y, et al. 297MO: Patterns of disease progression with durvalumab (D) after concurrent chemoradiotherapy (cCRT) in limited-stage small-cell lung cancer (LS-SCLC): Results from ADRIATIC. Journal of Thoracic Oncology. 2025 Mar 1;20(3, Supplement 1):S181–2.
- 3. Cheng Y, Spigel DR, Cho BC, Laktionov KK, Fang J, Chen Y, et al. Durvalumab after Chemoradiotherapy in Limited-Stage Small-Cell Lung Cancer. N Engl J Med. 2024 Oct 10;391(14):1313–27.
- 4. Levy A, Berghmans T, Koller M, Fournier B, Mauer M, Andratschke N, et al. PRIMALung (EORTC-1901): Prophylactic cerebral irradiation (PCI) or active brain magnetic resonance imaging (MRI) surveillance in small-cell lung cancer (SCLC) patients. Lung Cancer. 2024 Dec;198:107993.










