Stage IIIA NSCLC: A Review of Recent and Ongoing Trials
Mariano ProvencioTrial data can help inform change to standard of care, but at what point do we say enough data is enough and move forward with combination therapy as the new standard of care? Read more
Building and Maintaining a Patient–Physician Relationship in the COVID-19 Era
Robert Ramierz, DOAs oncologists, we recognize that the patient–physician relationship is of utmost importance in establishing the patient’s trust in the physician and in the physician’s ability to learn more about the patient’s needs and perform accurate assessments for […] Read more
Multidisciplinary Tumor Board: A Single Institution’s Perspective
Ari Rosenberg, MDAs 7:00 AM approaches, the ninth-floor conference room slowly fills as team members fi le in and find their seats. The senior radiation oncologist identifies his seat in the front row, carefully placing his stack […] Read more
Dr. Harpreet Singh Discusses the Conduct of FDA Clinical Trials During the COVID-19 Pandemic
Harpreet Singh, MDHarpreet Singh, MD, is director of Division of Oncology 2 at the U.S. Food and Drug Administration (FDA). In the following interview, she discusses the FDA’s response to the COVID-19 […] Read more
Coronavirus disease-2019 (COVID-19) is a novel infectious disease, mainly affecting the respiratory tract, caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV2). COVID-19 emerged in Wuhan, China, in December […] Read more
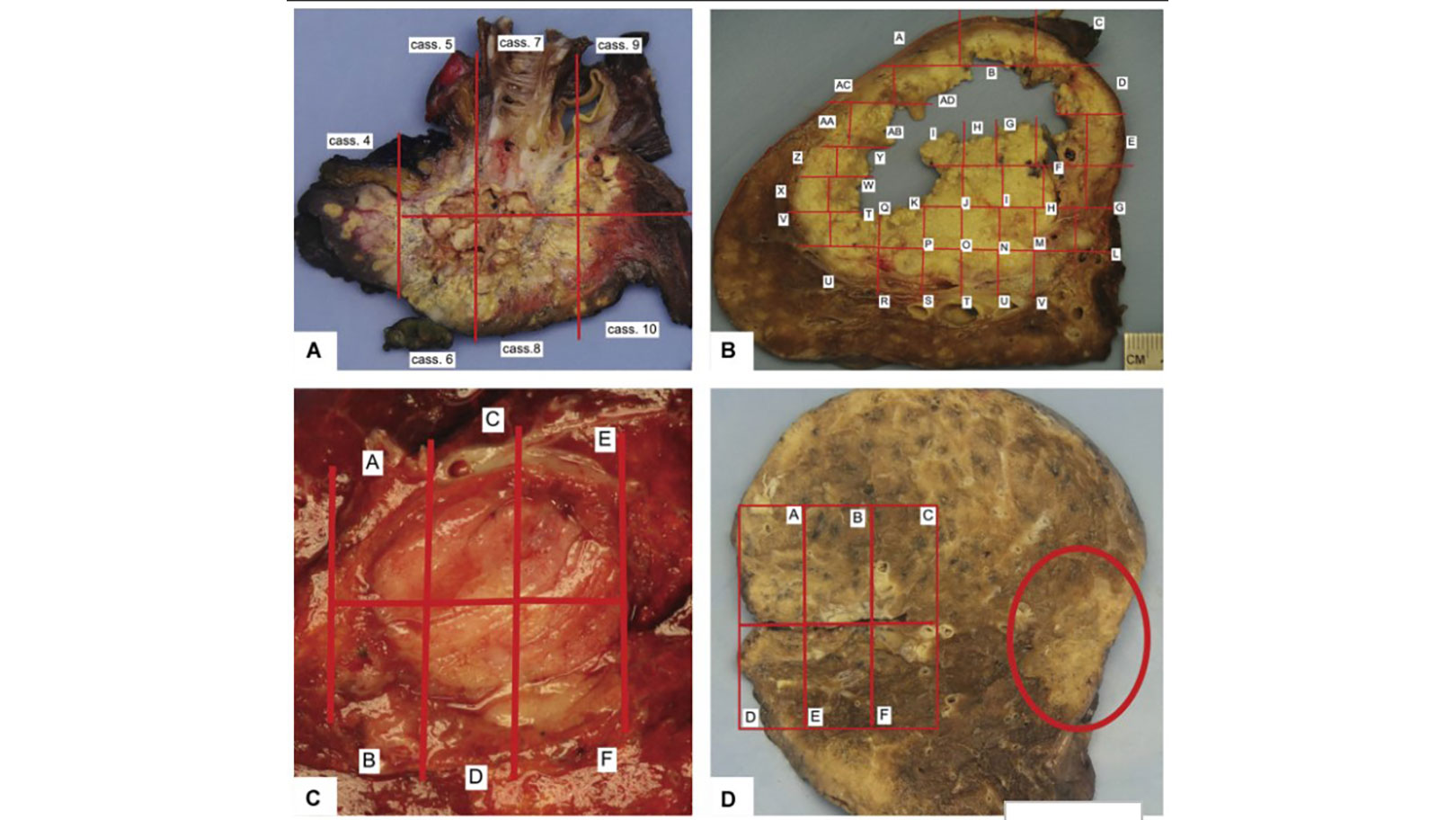
IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens Following Neoadjuvant Therapies
Sanja Dacic+more
Treatment guidelines recommend neoadjuvant or adjuvant therapies with surgery for patients with large primary tumors or clinical evidence of intrathoracic nodal spread.1,2 A recent joint IASLC–U.S. Food and Drug Administration […] Read more
The RAMES Study: A New Strategy of Targeting Angiogenesis in Mesothelioma
Federica Grosso, MD+more
At this year’s American Society of Clinical Oncology Virtual Meeting, the final results of the RAMES study were presented.1 RAMES was a prospective, placebo-controlled, phase II, randomized study in which […] Read more
Elimination of Clinical Trial Disparities Requires a New Lens for Viewing Justice, New Approach to Patient’s Role
By Joy Curzio Although there has been tremendous progress in lung cancer over the past 10 years, with a number of new drug indications being approved in the United States […] Read more
Immunotherapy, TKIs for Resectable/Resected NSCLC: Dr. Masahiro Tsuboi Provides a Comprehensive Overview of Promising Phase II Data, Phase III Status
By Jill Daigneault, PhD Surgical outcomes for early-stage NSCLC are still very poor due to the high rate of recurrence through distant metastases. It is likely that these distant metastases […] Read more
First-line Sintilimab Plus Platinum-Based Chemotherapy Prolongs PFS in Advanced Nonsquamous NSCLC
By Kara Nyberg, PhD Adding sintilimab, an anti-PD-1 antibody, to first-line platinum-based chemotherapy enhances efficacy in patients with locally advanced or metastatic nonsquamous NSCLC. In the randomized, placebo-controlled, phase III […] Read more