Despite U.S. Food and Drug Administration (FDA) approvals for the addition of anti–programmed death-ligand 1
(PD-L1) therapies to a first-line combination of etoposide plus a platinum-based agent for extensive-stage small cell lung cancer (SCLC).1
,2
Overall survival (OS) remains poor—roughly 1 year for patients diagnosed with extensive-stage disease. Promising new agents in relapsed disease, such as the recently FDA-approved alkylating agent lurbinectedin, have generated optimism, but it is not yet clear if such therapies will meaningfully affect patient survival.3
These modest gains serve to underscore the unmet needs for this devastating disease—namely, a lack of novel, active therapies to counteract chemotherapy and immunotherapy resistance and a lack of predictive biomarkers on which to base treatment decisions. Recent strides in the molecular characterization of the SCLC landscape, including the identification of transcriptional subtypes with unique therapeutic vulnerabilities, may finally yield an opportunity to meet these needs.
One promising class of targets in SCLC is DNA damage-repair proteins, including poly (ADP-ribose) polymerase (PARP), which are highly expressed in SCLC; preclinically, we have seen remarkable antitumor activity when DNA damage-repair proteins are inhibited in SCLC models.4
Clinically, PARP inhibitors have shown promise in SCLC, including two trials demonstrating objective response rates of approximately 40% when they were used in combination with the oral chemotherapeutic temozolomide for patients with relapsed SCLC.5
,6
In trials evaluating progression free survival (PFS) and OS in unselected populations, however, PARP inhibitors have not yet yielded significant improvements.
Strategies to enrich for PARP-inhibitor sensitivity via rational patient selection may be essential to surmount the absence of survival benefit observed to date. Several groups have identified the expression of the RNA/DNA helicase Schlafen-11 (SLFN11) as a putative predictive biomarker of PARP-inhibitor sensitivity in preclinical SCLC models.7
,8
,9
In one of the aforementioned trials that assessed a PARP inhibitor (veliparib) and temozolomide in combination for patients with relapsed SCLC, archival tissue samples underwent immunohistochemistry assessment of SLFN11 expression to evaluate this hypothesis.6
The analysis found that patients with SLFN11-positive disease who received veliparib had statistically significant improvements in PFS and OS compared with the patients who had SLFN11-negative disease—a feature not seen in the veliparib-free placebo arm.
Beyond SLFN11, the delineation of unique molecular subtypes of SCLC based on transcriptional profiling has generated compelling hypotheses for patient selection and assignment to multiple therapies, including PARP inhibitors (Figure).10
,11
On the basis of transcription factor expression, SCLC can be subdivided into ASCL1-high (SCLC-A), NEUROD1-high (SCLC-N), and POU2F3-high (SCLC-P) subtypes. A fourth subtype, SCLC-inflamed (SCLC-I), has low expression of all three transcription factors but increased infiltration by immune cells and high expression of immune checkpoints and inflammatory signatures. Each subtype features distinct biological attributes and therapeutic vulnerabilities in preclinical models. The SCLC-P subtype, for example, is exquisitely sensitive to PARP inhibitors independent of SLFN11 expression, whereas the SCLC-A subtype bimodally expresses SLFN11 and, therefore, has bimodal sensitivity to PARP inhibitors. Meanwhile, when considering the IMpower133 regimen, patients with the SCLC-I subtype experienced a near doubling of median OS when the anti-PD-L1 agent atezolizumab was added to carboplatin and etoposide (18.2 months vs. 10.4 months), whereas lesser benefits were observed for SCLC-A, SCLC-N, and SCLC-P (10.9, 10.6, and 9.6 months, respectively, vs. 10.6, 9.4, and 6.0 months, respectively).
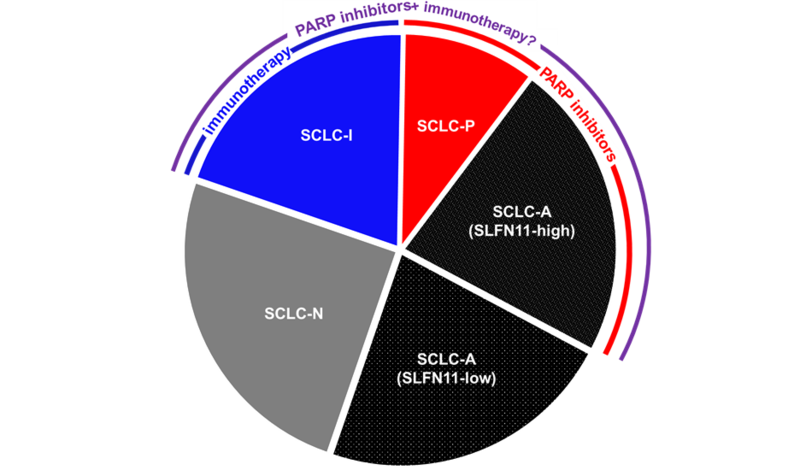
Because the other SCLC subtypes outside of the SCLC-I group, experienced only modest benefit from the addition of immunotherapy, investigators must shift their focus to enhancing immunotherapy response among these subtypes. In SCLC models, PARP inhibitors have demonstrated the capacity to do precisely this. Specifically, treatment of immunotherapy-resistant genetically engineered SCLC mouse models with a PARP inhibitor/immunotherapy combination resulted in dramatic and durable responses.12
Mechanistically, PARP inhibition increases DNA damage and the presence of cytosolic DNA, which in turn activates the cyclic pathway of GMP-AMP synthase (cGAS), stimulating interferon genes (STING). As a result of cGAS/STING pathway activation, tumors experience increased expression of immune checkpoints, including PD-L1, and increased infiltration by T cells.
Clinical efforts to combine PARP inhibitors and immunotherapy have included only small, early-phase trials to date. The phase I/II MEDIOLA trial (NCT02734004) in advanced solid tumors, which included a cohort with SCLC, investigated the combination of olaparib and durvalumab.13
Overall, the combination proved feasible, with a toxicity profile consistent with the combined monotherapy effects of olaparib and durvalumab. This trial unfortunately failed to meet prespecified median PFS and disease control-rate targets, but it may have been hampered by its design: the inclusion of a PARP-inhibitor monotherapy run-in resulted in disease progression in 18 of 38 total patients prior to the actual administration of the experimental combination. A separate, single-arm phase II trial evaluating the efficacy of olaparib and durvalumab in 19 patients with recurrent SCLC yielded objective response rate of only 10.5% and a median OS of just 4.1 months.14
However, 60% of the patients enrolled in this trial had platinum-refractory disease, which is known to correlate with PARP-inhibitor resistance.7
Nevertheless, several provocative observations from this small study suggest that the combination warrants further investigation. Both patients who had inflamed phenotypes at baseline, measured by high CD8-positive T-cell infiltration, had confirmed responses, and both of these patients also demonstrated increased PD-L1 expression on post-treatment biopsies, consistent with the proposed mechanism of PARP inhibitor/immunotherapy synergy.11
The path forward for PARP inhibitors with or without immunotherapy will depend on investigators’ abilities to select those patients most likely to benefit. Clinical trials already underway suggest this lesson has been learned. The SWOG1929 trial, recently activated nationwide, will randomly assign patients with SLFN11-positive disease (determined by immunohistochemistry) after first-line carboplatin/etoposide/atezolizumab to either single-agent atezolizumab maintenance (standard of care) or atezolizumab plus the PARP inhibitor talazoparib. An investigator-initiated trial (PRIO: NCT0472830) will also integrate a PARP inhibitor into the maintenance setting, in this instance with the anti–PD-L1 drug durvalumab as well as consolidative thoracic radiation, and will include both pre– and post–PARP inhibitor biopsies. This tissue collection strategy has also been integrated into an investigator-initiated trial for relapsed SCLC (NCT04701307) that pairs the PARP inhibitor niraparib with the anti–PD-1 dostarlimab.
Will prospective-patient selection using SLFN11 successfully target those most likely to benefit from PARP-inhibitor combinations? Will earlier integration of PARP inhibitors into the maintenance setting mitigate the overlapping resistance observed with platinum-based chemotherapy? Will pre- and post-treatment biopsies confirm predictions related to SCLC molecular subtype and combination PARP inhibitor/immunotherapy response? These ongoing trials promise much-needed answers to these questions regarding the viability of PARP inhibitors for SCLC.
Conflict of Interest Disclosures:
Dr. Negrao: Research funding to institution: Mirati, Novartis, Checkmate, Ziopharm, AstraZeneca, Pfizer; Consultant/Advisory Board: Mirati
Dr. Gay: Research funding to institution: AstraZeneca; Consultant/Advisory Board: Jazz Pharmaceuticals, Kisoji Biotechnology
Dr. Byers: Advisor/Consultant AND Research Funding: AstraZeneca, GenMab, Sierra Oncology; Research Funding: Tolero Pharmaceuticals; Consultant/Advisory Board: PharmaMar, AbbVie, Bristol-Myers Squibb, Alethia, Merck, Pfizer, Jazz Pharmaceuticals, Genentech, Debiopharm Group
- 1. a. b. Horn L, Mansfield AS, Szczęsna A, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med. 2018;379(23):2220-2229.
- 2. Paz-Ares L, Dvorkin M, Chen Y, et al. Durvalumab plus platinum-etoposide versus platinum-etoposide in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): a randomised, controlled, open-label, phase 3 trial. Lancet. 2019;394(10212):1929-1939.
- 3. Trigo J, Subbiah V, Besse B, et al. Lurbinectedin as second-line treatment for patients with small-cell lung cancer: a single-arm, open-label, phase 2 basket trial. Lancet Oncol. 2020;21(5):645-654.
- 4. Byers LA, Wang J, Nilsson MB, et al. Proteomic profiling identifies dysregulated pathways in small cell lung cancer and novel therapeutic targets including PARP1. Cancer Discov. 2012;2(9):798-811.
- 5. Farago AF, Yeap BY, Stanzione M, et al. Combination Olaparib and Temozolomide in Relapsed Small Cell Lung Cancer. Cancer Discov. 2019;9(10):1372-1387.
- 6. a. b. Pietanza MC, Waqar SN, Krug LM, et al. Randomized, Double-Blind, Phase II Study of Temozolomide in Combination With Either Veliparib or Placebo in Patients With Relapsed-Sensitive or Refractory Small-Cell Lung Cancer. J Clin Oncol. 2018;36(23):2386-2394.
- 7. a. b. Allison Stewart C, Tong P, Cardnell RJ, et al. Dynamic variations in epithelial-to-mesenchymal transition (EMT), ATM, and SLFN11 govern response to PARP inhibitors and cisplatin in small cell lung cancer. Oncotarget. 2017;8(17):28575-28587.
- 8. Lok BH, Gardner EE, Schneeberger VE, et al. PARP Inhibitor Activity Correlates with SLFN11 Expression and Demonstrates Synergy with Temozolomide in Small Cell Lung Cancer. Clin Cancer Res. 2017;23(2):523-535.
- 9. Murai J, Feng Y, Yu GK, et al. Resistance to PARP inhibitors by SLFN11 inactivation can be overcome by ATR inhibition. Oncotarget. 2016;7(47):76534-76550.
- 10. Gay CM, Allison Stewart C, Park EM, et al. Patterns of transcription factor programs and immune pathway activation define four major subtypes of SCLC with distinct therapeutic vulnerabilities. Cancer Cell. 2021;39(3):346-360
- 11. a. b. Frese KK, Simpson KL, Dive C. Small cell lung cancer enters the era of precision medicine. Cancer Cell.. 2021 Mar 8;39(3):297-299.
- 12. Sen T, Rodriguez BL, Chen L, et al. Targeting DNA Damage Response Promotes Antitumor Immunity Through STING-Mediated T-cell Activation in Small Cell Lung Cancer. Cancer Discov. 2019;9(5):646-661.
- 13. Krebs M, Ross K, Kim S, et al. P1.15-004 An Open-Label, Multitumor Phase II Basket Study of Olaparib and Durvalumab (MEDIOLA): Results in Patients with Relapsed SCLC. J Thor Oncol. 2017;12(11)(suppl 2):S2044-S2045.
- 14. Thomas A, Vilimas R, Trindade C, et al. Durvalumab in Combination with Olaparib in Patients with Relapsed SCLC: Results from a Phase II Study. J Thorac Oncol. 2019;14(8):1447-1457.






