
Joe Y. Chang, MD, PhD
The revised STARS trial1 was conducted in an effort to examine the long-term outcomes of stereotactic ablative radiotherapy (SABR) for patients with operable stage IA NSCLC and to compare these outcomes with a contemporary cohort of patients undergoing video-assisted thoracoscopic surgical lobectomy with mediastinal lymph node dissection (VATS L-MLND). The result showed almost identical overall survival (OS) and cancer-specific survival rates, with far fewer side effects and complications with SABR.
However, isolated regional recurrence occurred about 10% more often with SABR compared to VATS L-MLND, and lobar recurrence was also documented (6.3%). Managing post-SABR recurrences requires further discussion if SABR is to be increasingly utilized in the operable population.
Although the rate of isolated regional recurrence after SABR for a similar patient population is relatively low2, the findings of STARS were not surprising. The risk of occult untreated nodal disease was naturally higher in the SABR population because both propensity-matched cohorts included clinical stage I NSCLC, and MLND is not performed in patients who undergo SABR. This is because the discovery of nodal involvement during VATS L-MLND (10% in the revised STARS trial) allows for prompt administration of adjuvant chemotherapy. One hundred percent of such patients received chemotherapy in the trial, while 75% of those with pN2 cases received radiotherapy. Because this finding is specific to the inherent nature of an operative procedure compared with a nonoperative procedure, it strongly implies that patients who are eligible for SABR and who have operable disease must undergo thorough full disease staging—with any suspicious lymph nodes sampled by endobronchial ultrasound (EBUS)—and should be followed in a distinct manner. This is especially important for larger tumors and/or centrally located lesions.

Vivek Verma, MD
Most patients who undergo SABR are medically inoperable because of significant comorbidities, so their life expectancies are considerably limited compared with operable patients. Because death is a competing risk for recurrence, late recurrences may not be a major consideration when scheduling follow-up for medically inoperable patients. However, in the operable population, follow-up must continue regularly even through more distant time periods.
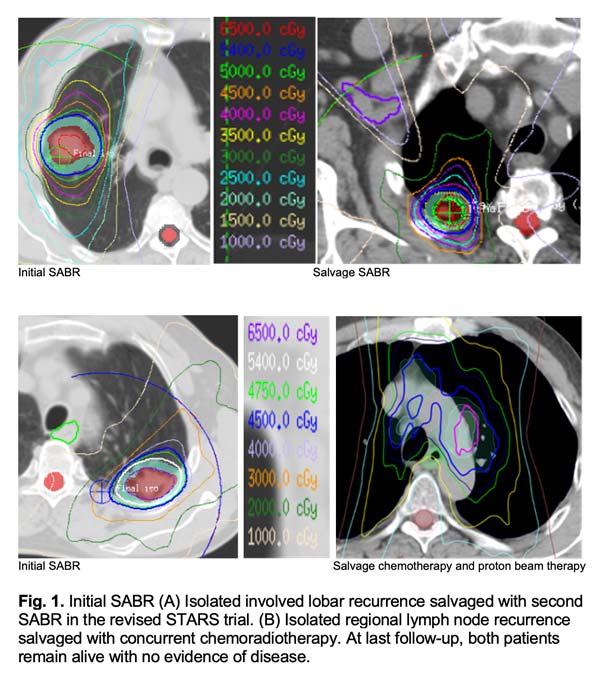
Prior to the revised STARS trial, long-term post-SABR patterns of failure had not been well evaluated in the operable population. The revised STARS trial stipulated clinical visits with chest CT every 3 months for the first 2 years, then every 6 months for the next 3 years, and annually thereafter. Individualized follow-up schedules based on tumor size, histology, and location merits exploration in the future. The purpose of closer follow-up of higher-risk cases directly relates to the potential for salvage therapy. Adequate salvage of isolated involved lobar recurrences (Fig. 1A) or regional lymph node recurrence (Fig. 1B) can lead to improved outcomes and a potential cure3,4, which likely explains why there were no differences in long-term survival despite a higher rate of regional recurrence in the SABR arm.
Local recurrence of irradiated lesion after SABR is also relatively rare (1.3% in revised STARS)2, but is difficult to diagnose because of the appearance of post-SABR inflammation and/or fibrosis, which may “hide” recurrence, thereby reducing the sensitivity of CT and/or PET imaging for accurate detection of local failure. In the revised STARS trial, systematic imaging review was conducted for all suspected recurrences. Any suspicious CT finding was evaluated by PET/CT imaging, and a biopsy was highly recommended to confirm recurrence.
To implement these same procedures at nonquaternary care centers, we recommend personalized systematic imaging review using dedicated principles established in the literature5. The rate of false positives on PET/CT in the irradiated area is high within 6 months after SABR, and cases with a maximum standardized uptake value (SUV) above 5 occurring 6 months or more after SABR should undergo biopsy to confirm recurrence if salvage therapy is planned6. Unlike the case for many medically inoperable patients, obtaining biopsies to evaluate for recurrences is easier in the operable population. However, biopsy of irradiated lesions within 3 months following SABR is not recommended because the ongoing process of cancer cell death and immune infiltration can result in a high false-positive rate. Additionally, if the bronchial tree has been irradiated to a large extent, EBUS should avoid these areas to prevent fistula due to compromised healing.
Moreover, reducing the likelihood for ambiguity in identifying a local recurrence requires efforts to reduce the baseline local recurrence rate. In addition to respiratory motion management and high-quality volumetric image guidance, when safe to deliver, we also recommend escalating the biologically effective dose (BED) of SABR using the paradigms set forth in the revised STARS trial, wherein the planning target volume (PTV) mean dose received a BED of greater than 130 Gy10 and the gross tumor volume (GTV) received a BED of 150 Gy10. Attention to treatment planning parameters when prescribing this dose is also highly consequential because failure to do so may increase the risk of local therapeutic failure7. Implementing such measures inherently reduces the risk that a suspected local failure is an actual local failure.
In summary, practical aspects of clinically managing operable SABR patients are important for the long-term care of this unique population. It is imperative to conduct adequate and individualized staging work-up, along with long-term follow-up of these patients to allow for the delivery of appropriate salvage therapies should an isolated local-regional recurrence be identified. Because these patents are expected to survive considerably longer than the medically inoperable population (both at baseline and following salvage), further work is needed to refine the nuances of managing the operable SABR population.
References
- 1. Chang JY, Mehran RJ, Feng L, et al. Stereotactic ablative radiotherapy for operable stage I non-small-cell lung cancer (revised STARS): long-term results of a single-arm, prospective trial with prespecified comparison to surgery. Lancet Oncol. 2021;22(10):1448-1457.
- 2. Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13(8):802-809.
- 3. Brooks ED, Sun B, Feng L, et al. Association of Long-term Outcomes and Survival With Multidisciplinary Salvage Treatment for Local and Regional Recurrence After Stereotactic Ablative Radiotherapy for Early-Stage Lung Cancer. JAMA Netw Open. 2018;1(4):e181390.
- 4. Brooks ED, Verma V, Senan S, et al. Salvage Therapy for Locoregional Recurrence After Stereotactic Ablative Radiotherapy for Early-Stage NSCLC. J Thorac Oncol. 2020;15(2):176-189.
- 5. Huang K, Senthi S, Palma DA, et al. High-risk CT features for detection of local recurrence after stereotactic ablative radiotherapy for lung cancer. Radiother Oncol. 2013;109(1):51-57.
- 6. Zhang X, Liu H, Balter P, et al, Positron emission tomography for assessing local failure after stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;85(5):1558-1565.
- 7. Zhao L, Zhou S, Balter P, et al. Planning Target Volume D95 and Mean Dose Should Be Considered for Optimal Local Control for Stereotactic Ablative Radiation Therapy. Int J Radiat Oncol Biol Phys. 2016;95(4):1226-1235.





