A leading cause of mortality worldwide, cancer was tied to nearly 10 million deaths in 2020.1
Lung cancer has remained unchallenged for more than 30 years as the most commonly diagnosed malignancy and the leading cause of cancer mortality worldwide, accounting for up to 1.8 million deaths per year.2
,3
Treatment for lung cancer depends mainly on histologic subtype, stage of disease, and patient performance status. In spite of this, radiation therapy (RT) will be used—at least once—for up to 50% of patients.4
Thus, RT is an essential component of contemporary lung cancer treatment, in both the curative and palliative settings. In recent years, improved diagnostic workup, such as low-dose CT scan, PET/CT scan, and MRI, along with the robust progress of immuno-oncology in lung cancer, has consistently led to improved long-term survival. Therefore, the prevalence of patients living with lung cancer is on the rise. For these patients, RT has become a cornerstone in the management of oligorecurrence or oligoprogressive disease, thus broadening the indications for RT over the last decade. This applies particularly to the use of novel techniques such as stereotactic body radiation therapy, which offers excellent local control, toxicity ranging from null to acceptable, and the potential to enhance survival in patients with oligometastasis.5
,6
In addition to its increasing use and evidence that it can improve prognosis, RT’s pricing has decreased in the past decade so that it now represents only about 5% of the total cost of cancer care.7
For instance, it is significantly less expensive than the novel immunotherapy agents a patient with lung cancer may also receive: A stereotactic body radiation therapy treatment in the United States8
averages $27,145 and is usually performed once, whereas the list price of a single dose of pembrolizumab, which would need to be repeated every 3 weeks until disease progression or for 2 years total, averages $10,067 per cycle. As a result, RT is considered by many to be a highly cost-effective component of the lung cancer treatment toolbox.
Nevertheless, investment in RT equipment worldwide is nearly nonexistent, representing under 10% of all the money budgeted for oncologic research, development, and clinical expenditure. Moreover, despite being a cost-effective treatment technique that is broadly used in a disease of increasing incidence and prevalence, RT is inadequately distributed across the globe. Access to RT is oddly low worldwide, but especially in low- to middle-income countries (LMICs) compared with developed nations. Developing countries have 85% of the world’s population, but only one-third of its RT equipment. It has been previously reported that more than half of African countries (29, all sub-Saharan) lack RT facilities, and that 12 countries in Latin America and the Caribbean (all of them small islands) lack this equipment.9
The International Atomic Energy Agency (IAEA) currently recommends that developed countries have four radiotherapy machines per every million inhabitants and that LMICs have up to 3.5 radiotherapy machines per every million inhabitants, a goal that is far from reality for basically all the LMICs.10
In the Latin America/Caribbean region, only 3 of 40 countries (Uruguay, Curaçao, and Antigua and Barbuda) meet that standard, and none of the African countries do. As stated in 2003 by Mohamed ElBaradei, IAEA’s then director-general, “A silent crisis in cancer treatment exists in developing countries, and it is intensifying every year”.11
In a 2016 paper, Yap et al.12
reported a deficit of approximately 7,100 RT megavoltage machines (MVMs) globally. Even in the best-case scenario and adjusted by the number of new patients per MVM per year, the deficit remains at 4,300 MVMs worldwide. Despite yearly increases in the availability of MVMs, requirements are not being met, and LMICs are clearly at a disadvantage. In that setting, more than 2 million patients with cancer are unable to access RT. That means that, in LMICs, only 5 of every 10 patients with cancer who need RT have access to it. This is troublesome, not only because access to treatment with this modality is insufficient, but also because RT’s indications continue to broaden in concert with the rise in the incidence and prevalence of cancer, particularly lung cancer.
Mexico—which has a higher standard of living than most LMICs—is no exception when it comes to this issue: The country, in which cancer is the third leading cause of death and lung cancer is the second most deadly form of the disease,3
does not meet the IAEA standard. According to the Organisation for Economic Co-operation and Development (OECD), the density of RT machines per million inhabitants in Mexico, whose population of 130 million is spread across 1.9 million km2
divided into 32 states, is 1.7 to 1.8. But in recent years, a diverse group of radiation oncologists in Mexico began to consider the accuracy of that estimate by addressing scientific evidence related to this problem. In 2020, the Mexican Radiation Oncology Certification Board (CMCRO) published a nationwide census13
of the country’s technological and human resources that demonstrated the staggering finding that the ratio of RT machines to Mexican citizens is even lower than the OECD’s estimate.
The results of the CMCRO survey can be used as a catalyst to secure funding for RT equipment, driving a positive change in patient care. The survey, which included the participation of 95.1% of Mexico’s 103 RT centers, showed a median of only two centers per state (ranging from 0 in Tlaxcala to 20 in Mexico City) and that 11 states (34.4%) had only one center. Most centers are public (56, or 54.3%), and only 14 (13.6%) have residency training programs: 12 radiation oncology residencies and 2 radiation oncology fellowships (1 in neurological radiosurgery and 1 in pediatric radiation oncology). The overall calculated density of RT machines per million inhabitants — according to the Mexican National Institute of Statistics and Geography’s last population register in 2015—is 1.32, ranging from 0 in Tlaxcala to 5.16 in Mexico City. The density of RT machines per million inhabitants is 0 in one state (3.1%), 0.1 to 0.49 in four states (12.5%), 0.5 to 0.99 in eight states (25%), 1 to 1.49 in nine states (28.1%), 1.5 to 1.99 in five states (15.6%), and two or more in five states (15.6%).
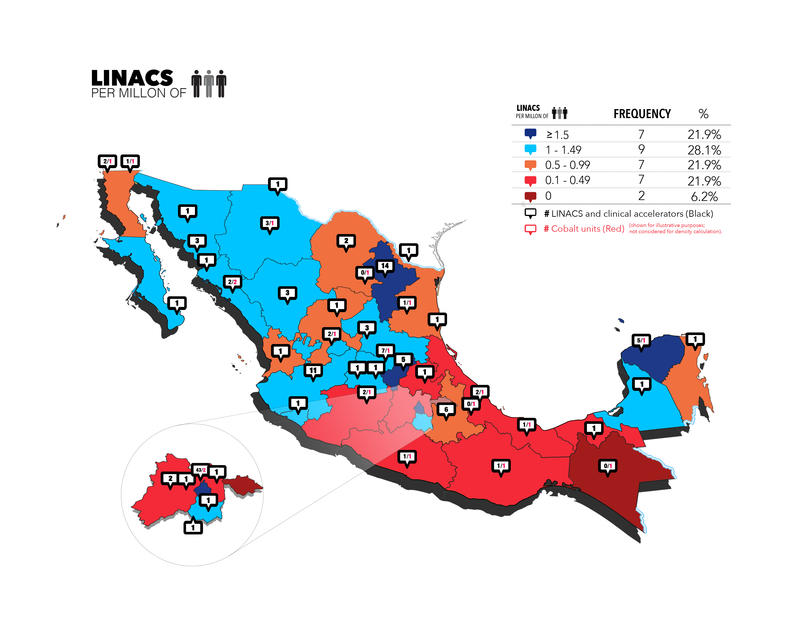
The census also looked at the number and type of RT machines in use in Mexico, which include external beam radiation therapy and brachytherapy (BT) technology. External beam radiation therapy employs devices that are situated at a distance from the patient, such as orthovoltage or supervoltage machines—specifically, cobalt machines and linear and clinical accelerators (such as cyber knife and tomotherapy). The census identified 141 linear and clinical accelerators throughout Mexico, representing 87% of its total number of RT machines (the remaining 13% are cobalt units, including two Gamma Knife machines). The density of linear and clinical accelerators per million people in each of Mexico’s 32 states is shown in Fig. 1, with an overall density in the country of 1.19 (ranging from 0 in Chiapas and Tlaxcala to 4.82 in Mexico City). However, Mexico is far from achieving IAEA recommendations. This ratio is 0 in two states (6.3%), 0.1 to 0.49 and 0.5 to 0.99 in seven states (21.9%) each, 1 to 1.49 in nine states (28.1%), and 1.5 or greater in seven states (21.9%). More than half the country’s accelerators, 77 (54.2%), are located in just four states (Mexico City, Nuevo León, Jalisco, and Guanajuato). Although still scarce, the number of high-precision accelerators has increased, according to previously published data.14
Figure 1
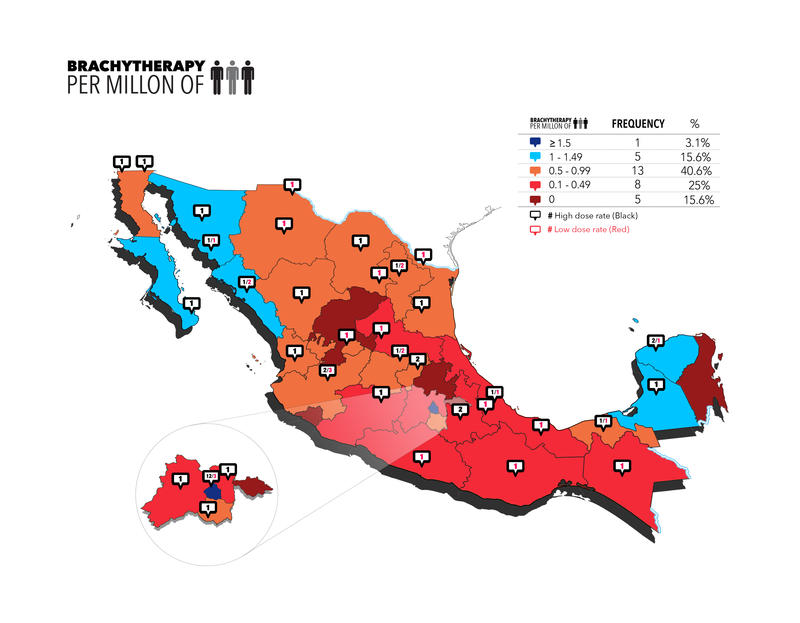
In the instance of BT, the radiation device is placed within, or close to, the target volume. Although mainly used to treat gynecologic tumors, the applications of this strategy have expanded widely. According to Mexican BT infrastructure records, only 59 of the 103 centers (57.3%) have a BT unit. The total number of BT units is 66, with a median of one center with a BT unit per state. There are 5 states with zero centers (15.6%), 11 states with one BT unit each (34.4%), 8 with two units each (25%), 5 with three machines each (15.6%), and 1 state each with 4, 5, and 15 BT units (3.1%). Thirty-seven of the machines provide automated afterload high-dose-rate BT (56.1%), whereas in 29 centers (43.9%) manual afterload low-dose-rate BT is still used.
The impact of the lack of RT is devastating. Delays in initiating and completing therapy and the absence of RT when indicated have deleterious implications on quality of life, local control, disease-free survival, and OS. This evidence and its implications can be extrapolated to patients who face barriers to accessing RT. Thus, the clinical and economic benefits of investment in RT are likely to be substantial, potentially helping to prevent the loss of productive years of life.
The survey provides useful insights about current radiation oncology capabilities in Mexico and the urgent need for the country and other LMICs to meet international standards by increasing access to RT, including newer technologies, and by training staff to treat patients who have cancer. One of the survey’s most important contributions is its provision of the scientific background needed to justify evidence-based investment and implementation of modern technology across the country. It invites our readers across both civil and governmental associations to reflect on their own countries’ capabilities, especially considering that the need for more RT facilities will skyrocket around the globe, particularly in LMICs, in the decade to come. It is necessary to estimate the demand for RT on a country-by-country basis, as this problem is far from exclusive to Mexico. While Mexican radiation oncology facilities are insufficient, together with those in Brazil they currently represent half the RT equipment in Latin America, a proportion that is steadily increasing.
The evidence put forth by the survey is solid: Treating cancer needs to be a global healthcare priority, and radiotherapy represents one of the most effective treatments, along with chemotherapy or surgery. It’s certain that the integration of adequate RT services in Mexico and other LMICs will depend, in large part, on the provision of more, and more appropriate, equipment. With greater quantity of precision equipment than ever in Mexico, the good news is that radionuclide therapy units such as cobalt-60 machines are being replaced by linear accelerators, 56% of BT units already employ the automated afterload high-dose rate, and human resources have been constantly growing in the last 10 years.
However, we must not overlook the importance of training human resources to support the application of these technologies. As such, the lack of sufficient training centers for these personnel in LMICs also contributes to the inaccessibility of RT as part of the integrated management strategy against cancer.
Figure 2
- 1. Ferlay J, Ervik M, Lam F, et al, eds. Cancer Today (Powered by GLOBOCAN 2018): IARC CancerBase No. 15. International Agency for Research on Cancer. 2018. Accessed February 2021. https://publications.iarc.fr/Databases/Iarc-Cancerbases/Cancer-Today-Po…
- 2. a. b. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136(5):E359-E386.
- 3. a. b. Arrieta O, Zatarain-Barron ZL, Aldaco F, et al. Lung Cancer in Mexico. J Thorac Oncol. 2019;14(10):1695-1700.
- 4. Barrón-Barrón F, Guzmán de Alba E, Alatorre-Alexander J, et al. National Clinical Practice Guidelines for the management of non-small cell lung cancer in early, locally advanced and metastatic stages. Salud Publica Mex. 2019;61(3):359-414.
- 5. Blake-Cerda M, Lozano-Ruiz F, Maldonado-Magos F, et al. Consolidative stereotactic ablative radiotherapy (SABR) to intrapulmonary lesions is associated with prolonged progression-free survival and overall survival in oligometastatic NSCLC patients: A prospective phase 2 study. Lung Cancer. 2021;152:119-126.
- 6. Arrieta O, Barrón F, Maldonado F, et al. Radical consolidative treatment provides a clinical benefit and long-term survival in patients with synchronous oligometastatic non-small cell lung cancer: A phase II study. Lung Cancer. 2019;130:67-75.
- 7. Ringborg U, Bergqvist D, Brorsson B, Cavallin-Ståhl E, Ceberg J, et al. The Swedish Council on Technology Assessment in Health Care: systematic overview of radiotherapy for cancer including a prospective survey of radiotherapy practice in Sweden 2001—summary and conclusions. Acta Oncol. 2003;42(5-6):357-365.
- 8. Halpern JA, Sedrakyan A, Hsu W-C. Utilization, Complications, and Costs of Stereotactic Body Radiation Therapy (SBRT) for Localized Prostate Cancer. Cancer. 2016;122(16):2496-2504.
- 9. Bishr MK, Zaghloul MS. Radiation Therapy Availability in Africa and Latin America: Two Models of Low and Middle Income Countries. Int J Radiat Oncol Biol Phys. 2018;102(3):490-498.
- 10. Rosenblatt E, Acun a O, Abdel-Wahab M. The challenge of global radiation therapy: An IAEA perspective. Int J Radiat Oncol Biol Phys. 2015;91(4):687-689.
- 11. IAEA.org. Millions of Cancer Victims in Developing Countries Lack Access to Life-Saving Radiotherapy. June 26, 2003. Updated February 16, 2018. Accessed June 12, 2021. https://www.iaea.org/newscenter/pressreleases/millions-cancer-victims-d…
- 12. Yap ML, Zubizarreta E, Bray F, Ferlay J, Barton M. Global Access to Radiotherapy Services: Have We Made Progress During the Past Decade? J Glob Oncol. 2016;2(4):207-215.
- 13. Maldonado Magos F, Lozano Ruiz FJ, Pérez Álvarez SI, et al. Radiation oncology in Mexico: Current status according to Mexico`s Radiation Oncology Certification Board. Rep Pract Oncol Radiother. 2020;25(5):840-845.
- 14. Poitevin-Chacón A, Hinojosa-Gómez J. Patterns of care of radiotherapy in México. Rep Pract Oncol Radiother. 2013;18(2):57-60.






