
Núria Rodriguez de Dios, MD, PhD
Núria Rodriguez de Dios, MD, PhD, is a radiation oncologist at Hospital del Mar, Hospital del Mar Medical Research Institute (IMIM), Pompeu Fabra University, in Barcelona, Spain. She talked with ILCN about the findings and implications of the PREMER study, a phase III trial comparing prophylactic cranial irradiation (PCI) with and without hippocampal avoidance in small cell lung cancer (SCLC).1
Q: What is the role of PCI in SCLC?
A: PCI confers an overall survival (OS) benefit in patients with limited-stage SCLC (LS-SCLC) and is considered standard of care for that disease. However, the role of PCI is not as well-defined in patients with stage I-II SCLC, who have a lower risk of developing brain metastases, or in those age 70 or older, or patients whose health is frail. In such cases, risk assessment should be individualized, and decision making should be shared with patients. In 2007, a European Organisation for Research and Treatment of Cancer (EORTC) trial,2 showed that PCI improved OS in patients with extensive-stage SCLC (ES-SCLC) who derived any benefit from first-line systemic therapy; thus, PCI became the standard of care in the ES-SCLC setting for a number of years. However, a 2017 randomized trial in Japan showed that PCI did not increase OS for patients with ES-SCLC.3 In this trial, disease stage and central nervous system (CNS) status for each patient was confirmed with MRI of the brain; patients without brain metastases were then randomly assigned to either PCI or observation. They underwent brain MRI every 3 months. The study was terminated early because of futility. Nevertheless, both studies1,2 showed a reduction in the incidence of brain metastases.
A consensus analysis by 13 experts from the European Society for Therapeutic Radiation Oncology and the International Association for the Study of Lung Cancer recommends PCI in selected patients and that its use be restricted primarily to fit, non-elderly patients who have responded to chemotherapy.4 The SWOG S1827 phase III (MAVERICK) trial (NCT04155034) is currently being performed in patients with both LS-SCLC and ES-SCLC to determine whether OS with MRI surveillance alone is not inferior to MRI surveillance plus PCI with or without hippocampal avoidance. The radiotherapy technique is chosen per patient and clinician discretion. A similar study is set up within the EORTC as well.
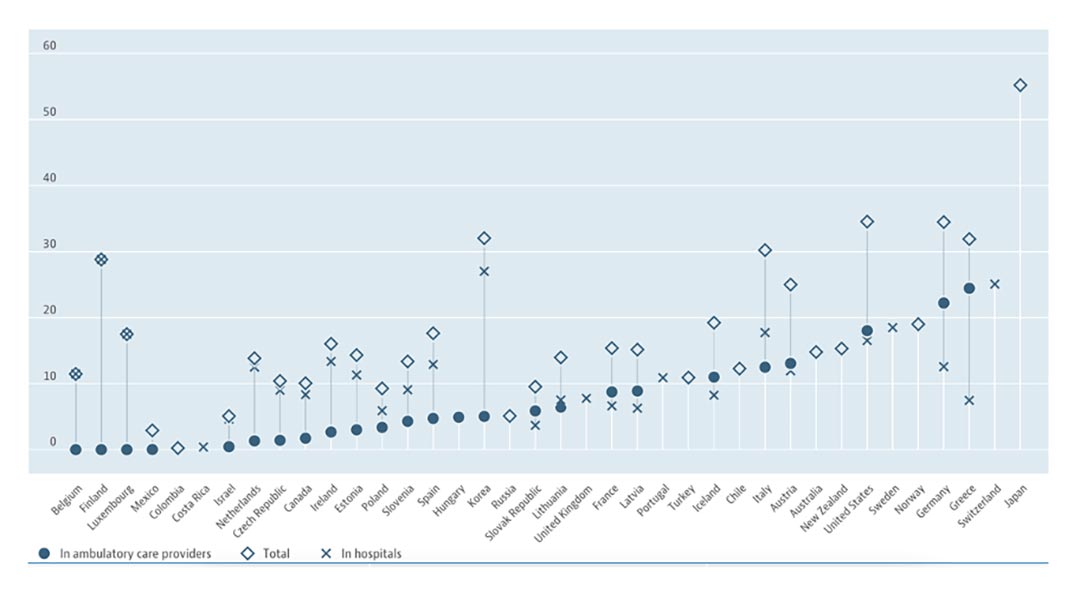
Fig. Number of MRI Units, by Country.
Per 1 million inhabitants, as of 2020 or latest available data. Reprinted with permission of OECD.
Q: Should PCI with hippocampal avoidance (HA-PCI) be considered the standard of care for patients with SCLC receiving PCI? Why or why not?
A: In the low-dose arm of the RTOG 0212 trial, more than 60% of patients receiving 25 Gy developed neurotoxicity at 1 year.5 Even at a large academic center, only a relatively small subset of patients—approximately half—with LS-SCLC who responded to initial chemoradiotherapy ultimately received PCI. The most common reasons for patient refusal were concerns about neurocognitive toxicity. Physicians also withhold PCI from a similar number of patients because of their own concerns regarding tolerability.6 Overall, these results suggest that efforts to decrease neurotoxicity of PCI may broaden the application of this intervention.
Two multicenter phase III trials assessing the benefits and risks of hippocampal preservation in SCLC patients receiving PCI have been published. A Netherlands Cancer Institute–Dutch Cancer Society trial did not show any difference in decline on the Hopkins Verbal Learning Test—Revised (HVLT–R) at 4 months compared with baseline.7 In contrast, the PREMER trial showed significantly less decline in delayed free recall (DFR) on the Free and Cued Selective Reminding Test (FCSRT) at 3 months in patients who received HA-PCI (5.8%) compared with those who received standard PCI (23.5%). Secondary endpoints included other FCSRT scores, quality of life (QoL), evaluation of the incidence and location of brain metastases, and OS. Data were recorded at baseline and 3, 6, 12, and 24 months after PCI. Analysis of all FCSRT scores showed a decline in the total recall (TR) at 3 months; DFR, TR, and total free recall (TFR) at 6 months; and TR at 24 months. The incidence of brain metastases, OS, and QoL were not significantly different.1
Despite the similarities, some methodological variations between the two studies might explain the difference in cognitive outcomes.8 The PREMER trial was powered to detect a 50% difference on the FCSRT delayed free recall measure at 3 months (a decline of greater than or equal to three points from baseline). The proportion of surviving patients providing data for the neurocognitive function in this study compares favorably when compared with other studies incorporating this endpoint. The compliance rate was lowest at 24 months for both arms; for the other time points, it ranged between 71.5% and 100.0%. A central review carried out within a few days of treatment captured meaningful feedback.
Many clinical trials of patients with brain metastases have found improved cognitive function and patient-reported outcomes with hippocampal avoidance added to whole-brain radiation.9,10 Furthermore, not only did the PREMER trial show improved cognitive outcomes, but both the PREMER trial and the Netherlands trial demonstrated the safety of HA-PCI with respect to survival and control of brain metastases. Given these findings, HA-PCI should be offered to any patient with SCLC who is a candidate for PCI.
One ongoing trial is NRG-CC003 (NCT02635009), which was recently expanded to 392 patients. The primary endpoint is HVLT-R delayed recall deterioration at 6 months (detecting a 14.5% absolute reduction in the probability of HVLT-R delayed recall deterioration due to HA-PCI suggests a 50% relative improvement), and it includes multidimensional cognitive testing that may facilitate a more comprehensive neurocognitive assessment of patients.
Q: Can you comment on the finding that no change in QoL was noted with HA-PCI?
A: The absence of differences in QoL may be related to one or more of the following reasons: The FCSRT is an objective test, whereas the EORTC Core Quality of Life Questionnaire C30 and the brain-specific BN20 questionnaire are self-reported and therefore subjective. The perception of a patient with lung cancer in the eyes of the general public is often of a patient in the final stage of their disease. When patients perform better than they would have expected in their daily life, they may report a better functional status on the QoL tests than they actually have.
Meyers et al.11 evaluated the neurocognitive endpoints for brain tumor clinical trials and concluded that self-report of cognitive problems correlated poorly with objective neurocognitive assessment. QoL changes did not parallel cognitive changes and thus could not be used as a proxy for neurocognitive assessment. QoL was also impacted by patients’ extracranial or systemic disease and other ongoing interventions or medications.
In patients with brain metastases who underwent whole-brain radiotherapy, Li et al.12 found that neurocognitive function declined before patient self-reported QoL and that memory function was the earliest sign of functional deterioration, whereas other neurocognitive domains such as executive function deteriorated later.
Lee et al.13 explored the benefits and risks of PCI in patients with LS-SCLC who have experienced complete response. They examined the benefit–risk ratio of PCI by varying the cure fraction, neurotoxicity (NT) rate, and the severity of NT. They constructed a decreasing QoL utility function. For the moderate NT rate: scenarios 1 (mild severity) and 2 (substantial severity) found that the average QoL utility decreased to 0.85 for the mild NT case and 0.70 for the substantial NT case at 15 years. The average utility decline in the first 2 years was minimal in all scenarios.
Q: How do the results from this trial put PCI for SCLC into perspective?
A: The results of the ongoing phase III trial S1827/MAVERICK could change current treatment of patients with SCLC. Patients are being stratified on the basis of stage (LS-SCLC versus ES-SCLC), usage of immunotherapy, and performance status, with a primary endpoint of OS. However, some considerations should be raised. First, routine brain MRI follow-up every 3 months could result in considerable socioeconomic impact, and may not be feasible in clinical practice in the majority of the world. For example, the availability of MRI in Europe is limited compared with nations such as Japan. As of 2019, Japan had the highest density of MRI units in the world. Over 55 such units were available per every million of its population. The United States and Germany followed with rates of about 35 per one million inhabitants. Compared to these countries, Israel and Hungary, for example, have around 5 MRI units per every million (Fig).14 Second, many patients undergoing MRI may have adverse experiences due to anxiety, claustrophobia, physical discomfort related to remaining still and supine for a relatively lengthy period of time, poor tolerance of scanner noise, or delays related to scheduling or safety screening for MRI, which are undesirable from the perspective of both patient experience and cost.15
Recently, Kim et al.16 performed a cost-effectiveness analysis comparing MRI surveillance alone with PCI for ES-SCLC. In the base case scenario, PCI was not cost-effective compared to MRI surveillance alone because of the impact of PCI on neurocognition decline, as estimated from available evidence. In a scenario analysis, when all patients receive HA-PCI, the model results become nearly cost-effective with PCI because of the presumed lower rate of cognitive decline (incremental cost-effectiveness ratio: $129,307 per quality-adjusted life-year gained); the ongoing MAVERICK trial may confirm this.
There are limited data on the integration of PCI and immunotherapy. In the IMpower-133 study, PCI was allowed in the maintenance phase, and 22 patients in each arm received this treatment.17 In the CASPIAN trial,18 PCI was allowed on the control arm, but only 7.9% of those patients received it. In the KEYNOTE-604,19 PCI was optional for both arms, and 11.8% and 14.2% of patients in the pembrolizumab and placebo arms, respectively, underwent PCI. However, no details of the toxicity related to PCI were provided in any of these studies. It will be interesting to analyze the impact of novel immunotherapy agents on the incidence of brain metastases and the relative effects of PCI in these patients. Trials are being launched to evaluate the brain activity of chemoimmunotherapy for patients with SCLC brain metastases (e.g., NCT04610684). However, other endpoints of neurologic death (NRG-BN009/NCT04588246) and neurocognitive function and patient-reported outcomes (NRG-CC003/NCT02635009 and NRG-CC009/NCT04804644) remain important and warrant continued study.
References
- 1. Rodríguez de Dios N, Couñago F, Murcia-Mejía M, et al. Randomized Phase III Trial of Prophylactic Cranial Irradiation With or Without Hippocampal Avoidance for Small-Cell Lung Cancer (PREMER): A GICOR-GOECP-SEOR Study. J Clin Oncol. 2021;39(28):3118-3127.
- 2. Slotman B, Faivre-Finn C, Kramer G, et al. Prophylactic cranial irradiation in extensive small-cell lung cancer. N Eng J Med. 2007;357(7):644-672.
- 3. Takahashi T, Yamanaka T, Seto T, et al. Prophylactic cranial irradiation versus observation in patients with extensive-disease small-cell lung cancer: a multicenter, randomized, open-label, phase 3 trial. Lancet. 2017;18(5):663-671.
- 4. Putora PM, Glatzer M, Belderbos J, et al. Prophylactic cranial irradiation in stage IV small cell lung cancer: Selection of patients amongst European IASLC and ESTRO experts. Radiother Oncol. 2019;133:163-166.
- 5. Wolfson AH, Bae K, Komaki R, et al. Primary analysis of a phase II randomized trial Radiation Therapy Oncology Group (RTOG) 0212: Impact of different total doses and schedules of prophylactic cranial irradiation on chronic neurotoxicity and quality of life for patients with limited-disease small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2011;81(1):77-84.
- 6. Lok BH, Ma J, Foster A, et al. Factors influencing the utilization of prophylactic cranial irradiation in patients with limited-stage small cell lung cancer. Adv Radiat Oncol. 2017;2: 548-554.
- 7. Belderbos J, De Ruysscher D, De Jaeger K, et al. Phase III trial of Prophylactic Cranial Irradiation With or Without Hippocampus Avoidance in SCLC (NCT01780675). J Thorac Oncol. 2021;16(5):840-849.
- 8. Brown PD, Parsons MW, Rusthoven CG, Gondi V. Hippocampal avoidance cranial irradiation: A New standard of care? J Clin Oncol. 2021;39:3093-3096.
- 9. Gondi V, Pugh SL, Tome WA, et al. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810-3816.
- 10. Brown PD, Gondi V, Pugh S, et al. Hippocampal avoidance during whole brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG Oncology CC001. J Clin Oncol. 2020;38:1019-1029.
- 11. Meyers CA, Rock RP, Fine HA. Refining endpoints in brain tumor clinical trials. J Neurooncol. 2012;108(2):227-230.
- 12. Li J, Bentzen SM, Li J, et al. Relationship between neurocognitive function and quality of life after whole-brain radiotherapy in patients with brain metastasis. Int J Radiat Oncol Biol Phys. 2008;71(1):64-70.
- 13. Lee JJ, Bekele BN, Zhou X, et al. Decision analysis for Prophylactic Cranial Irradiation for patients with small-cell lung cancer. J Clin Oncol. 2006;24(22):3597-3603.
- 14. OECD. Health at a Glance 2019: OECD Indicators. Accessed November 8, 2021. https://doi.org/10.1787/4dd50c09-en
- 15. Nguyen XV, Tahir S, Bresnahan BW, et al. Prevalence and financial impact of claustrophobia, anxiety, patient motion, and other patient events in magnetic resonance imaging. Top Magn Reson Imaging. 2020;29(3):125-130.
- 16. Kim H, Keller A, Beriwal S, Smith KJ, Vargo JA. Cost-Effectiveness of Prophylactic Cranial Irradiation Versus MRI Surveillance for Extensive-Stage Small Cell Lung Cancer. Int J Radiat Oncol Biol Phys. 2021. [Epub ahead of print].
- 17. Horn L, Mansfield AS, Szczęsna A, et al. First-Line Atezolizumab plus Chemotherapy in Extensive-Stage Small-Cell Lung Cancer. N Engl J Med. 2018;379(23):2220-2229.
- 18. Goldman JW, Dvorkin M, Chen Y, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol. 2021;22(1):51-65.
- 19. Rudin CM, Awad MM, Navarro A, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol. 2020;38(21):2369-2379.





