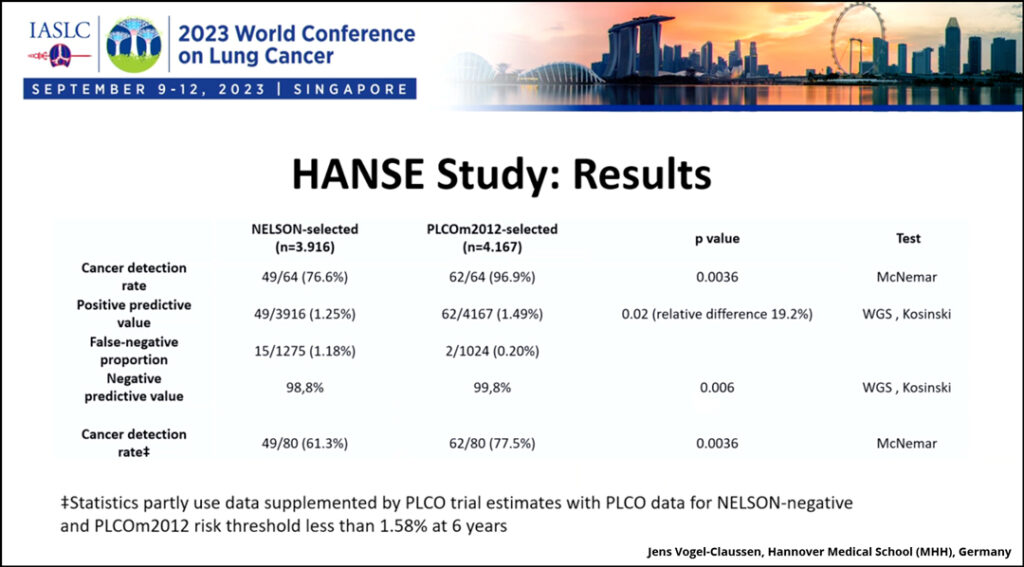
Baseline screening data from the German HANSE study demonstrates that the PLCOm2012 risk model1 had a superior positive predictive value for screen-detected lung cancers versus the NELSON criteria. The relative difference in cancer detection was 19% higher in the PLCOm2012-selected group, according to data presented at the 2023 World Conference on Lung Cancer in Singapore.

“Our data show that PLCOm2012 appears to be reliable and more efficient than the NELSON criteria for selecting individuals to be enrolled into a lung cancer-screening program,” said Jens Vogel-Claussen, MD, Professor and Vice Chair of the Department of Radiology at Hannover Medical School, Hanover, Germany. “The PLCO model should be used and preferred for identifying high-risk individuals based on our baseline results.”
Dr. Vogel-Claussen presented the data during an oral abstract session at WCLC 23. The session, Refining Risk Prediction Models for Lung Cancer Screening, is available on-demand for registered WCLC attendees until December 31.
The PLCOm2012 tool is a validated lung cancer risk prediction model based on data from the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO).2 Risk prediction models include individualized variables known to increase the risk for lung cancer in addition to the categorical variables of age and smoking history. The PLCOm2012 model variables include age, race/ethnicity, education level attained, body mass index, personal history of having a smoking-related cancer, family history of lung cancer, and personal history of having COPD or emphysema. The additional variables help to estimate the risk of lung cancer beyond determining if an individual is eligible for screening.
The HANSE study is a prospective, randomized, multicenter trial designed to answer critical open questions to facilitate the successful implementation of a structured low-dose computed tomography (LDCT) lung cancer screening program in Germany, as Germany does not currently have such a program. The use of LDCT for lung cancer screening has been shown in several studies to lead to earlier diagnosis and reduced mortality. However, Germany does not have a structured national lung cancer screening program using LDCT.
The HANSE study enrolled 5,191 high-risk participants who met either the NELSON risk criteria or the PLCOm2012 1.58% threshold risk within 6 years of screening. The NELSON risk criteria included people who currently smoke and those who previously smoked but who quit in the past 10 years and who smoked more than 15 cigarettes per day for more than 25 years or more than 10 cigarettes per day for more than 30 years. As previously mentioned, the PLCOm2012 risk model incorporates additional criteria beyond smoking history, including ethnicity, education, body mass index, preexisting COPD, and family history of lung cancer. Of the 5,191 high-risk participants, 4,170 fulfilled the PLCOm2012-selection criteria, and 3,920 fulfilled the NELSON-selection criteria.
The primary endpoint of the HANSE study was the comparison of the positive predictive value for lung cancers detected in PLCOM2012-selected versus NELSON-selected high-risk groups. Additional outcomes included the reliability of self-reported versus on-site, physician-assessed PLCOm2012 criteria and the positive predictive value of malignant versus benign tissue sampling.
Among the 5,191 patients enrolled, 64 cases of lung cancer were identified. Ninety-seven percent of the lung cancer cases were detected when PLCOm2012 model was applied, while only 77% were detected with the application of NELSON criteria. The two lung cancers missed by the PLCOm2012 model were stage 1 adenocarcinomas in two female participants.
“We reached the primary endpoint. The positive predictive value was 1.25% in the NELSON-selected group and 1.49% in the PLCOm2012-selected group; this difference was significant. The relative difference in cancers detected was 19% higher. This is higher than we estimated in our calculation,” Dr. Vogel-Claussen said. “This leads to a higher cancer detection rate, so higher sensitivity, which was significant, and a higher negative predictive value, which was also significant.”
PLCOm2012-selected participants were significantly older and had significantly more comorbidities than NELSON-selected participants, but there were no significant sex differences. Further, PLCOm2012-selected participants had higher cumulative estimated life expectancies than NELSON-selected participants (907 vs. 756 years). Follow-up outcome analyses will be conducted in years 1, 5, and 10.
An analysis of how the PLCOm2012 model compares to US Preventive Services Task Force criteria can be found here.
- 1. Tammemägi MC, Katki HA, Hocking WG, et al. Selection criteria for lung-cancer screening [published correction appears in N Engl J Med. 2013 Jul 25;369(4):394]. N Engl J Med. 2013;368(8):728-736. doi:10.1056/NEJMoa1211776
- 2. Gohagan JK, Prorok PC, Greenwald P, Kramer BS. The PLCO Cancer Screening Trial: Background, Goals, Organization, Operations, Results. Rev Recent Clin Trials. 2015;10(3):173-180. doi:10.2174/1574887110666150730123004