Lung cancer screening (LCS) is recommended for individuals at increased risk for lung cancer based on age and smoking history.1 LCS clinical trial data show that 50-55% of participants in these studies currently smoke cigarettes, and of those, up to 90% continue smoking after LCS.2 There is strong evidence that combining smoking cessation interventions with LCS is highly cost-effective, preventing deaths from lung cancer and other causes.3
Adding a cessation intervention with 15% abstinence effectiveness at the time of the first screen and assuming that 30% of eligible people undergo LCS is estimated to avert 2,422 additional lung cancer deaths in the 1960 US birth cohort.4 As the population ages, there will continue to be a demand for efficient and effective systems to offer smoking cessation on a large scale.5
Numerous cessation interventions and services have been shown to be effective globally.6 Until recently, it was unclear how to best design systems that could extend the reach of these treatments to maximize the integration of smoking cessation in the context of LCS.
Overview of the SCALE Trials
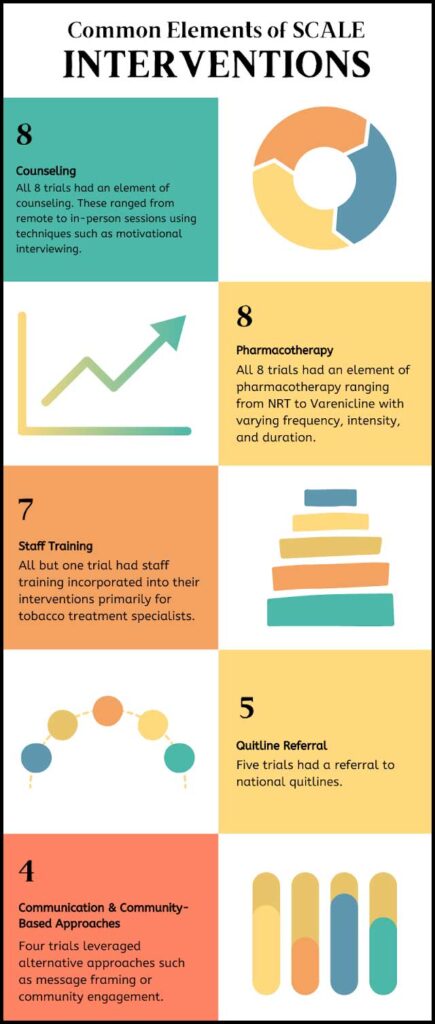
The Smoking Cessation at Lung Examination (SCALE) Collaboration comprises eight clinical trials, seven funded by the US National Cancer Institute and one by the US Veterans Health Administration (VA).5,7 This research sought to determine the key components and characteristics of successful smoking cessation programming at LCS. These trials developed and tested a variety of intervention combinations.
At a minimum, most of the SCALE trials included counseling (telephone and/or in-person), pharmacotherapy (such as nicotine replacement therapy [NRT] or prescribed medications), and staff training. Some trials also included technology-based interventions, quitline referrals, and community engagement. Collectively, the trials were conducted in 76 clinics with over 5,700 research participants nationwide.
SCALE Collaboration members developed a Special Measures Collection (SMC) of publicly available, curated measures of demographics, tobacco use, medical history, psychological variables, implementation, medical outcomes, and organizational characteristics. SMC data are shared across project teams. The Collaboration also created the Cessation Intervention Measurement Tool (CIMTool), which measures modalities, dosages, and durations of tobacco treatments.
Five of these trials have been published; 8,9,10,11,12,13,14,15,16 the remaining trial results are forthcoming.17,18,19,20
Published and Forthcoming Results
There was variability in quit rates across trials, with the most intensive protocols demonstrating stronger efficacy. For example, the trials conducted at MD Anderson Cancer Center (MDACC)14 and Memorial Sloan-Kettering Cancer Center (MSKCC)20 each achieved 30% quit rates (7-day point prevalence at the 6-month follow-up) with the most efficacious treatments.
The intensive treatments in these trials included integrated care: in-person and remote motivational and cognitive behavioral counseling for eight sessions and NRT or other pharmacotherapy for a 10- to 12-week period, with pharmacotherapy prescribed by dedicated tobacco treatment specialists at MDACC and a multiphase optimization strategy (multiple types of NRT for 6 weeks, two sessions of in-person or remote counseling using motivational interviewing, and message framing), at MSKCC.
The trial conducted at Massachusetts General Hospital also demonstrated higher 7-day tobacco abstinence at the 6-month follow-up (17.3% vs. 11.7%, p=0.045) for individuals offered eight counseling sessions over 12 weeks versus four sessions over 4 weeks.15 Its centralized tobacco cessation program provided counseling via video and phone. Abstinence was not significantly different by duration of NRT (2 weeks vs. 8 weeks of NRT patches). Interest in quitting smoking was not an eligibility requirement for this study.
A trial conducted by Georgetown University tested eight sessions of telephone counseling and 8 weeks of NRT patches without other pharmacotherapy.⁸ Cessation rates of 14.3% at 3 months and 10.3% at 6 months are encouraging given that 48% of participants in the trial were not yet ready to quit at the time of enrollment.
A University of Minnesota trial also yielded promising quit rates, using an unusually rigorous endpoint: 6 months of sustained abstinence at the 18-month follow-up. This trial provided phone coaching and NRT for 1 year and randomized participants based on an early (4- or 8-week) abstinence assessment into either more or less intensive treatments.
Although the efficacy rates of the randomized treatment groups were not significantly different, both groups achieved quit rates of 25% among initially abstinent participants and 16-18% among those who were non-abstinent at the early assessment.13
Some less-intensive protocols also demonstrated promising results; a 28% quit rate was observed at 6 months for quitline referrals and combinations of NRT or other pharmacotherapies for up to 12 weeks prescribed by the LCS providers in the MDACC trial.
The SCALE trials also demonstrated that the least intensive interventions may not be adequate for this population of people who have smoked for many years or who are less ready to set a quit date. For example, the VA trial conducted at the University of Washington did not find significant differences between the standard of care and the trial intervention, perhaps due to the low uptake of behavioral counseling (14% of intervention participants).18
The trial at the Medical University of South Carolina tested a lower-intensity intervention of messaging plus a 2-week starter package of NRT and did not find significant improvements in the quit rate.19
Factors that promoted success included having an accessible navigator or a tobacco cessation champion within the LCS setting. This was demonstrated in the effectiveness-implementation cluster randomized trial conducted at the Wake Forest University. In this trial a low-cost package of training and support was delivered to clinical providers; of note, the research team did not intervene directly with patients.11 This trial focused on building the capacity of a health system and imaging setting staff to offer cessation support using resources already available within the system.
The intervention and control groups did not differ significantly with respect to patient quit rates, but there was considerable variation across clinics; quit rates averaged 13% but ranged up to 27%, providing data to better understand the drivers of success.
Next Steps and New Opportunities
Smoking cessation services should be broadly integrated into the LCS clinical process. The results suggest that pharmacotherapy may be necessary, but it is not sufficient without additional support (e.g., counseling, referrals, training). It is important to recognize that in SCALE trials, every participant received some form of intervention.
Null findings mean only that the more intensive treatment was not more efficacious; they should not be interpreted to mean that providing cessation services with LCS is not worthwhile. The SCALE Collaboration is demonstrating feasible approaches to providing smoking cessation with LCS and producing a set of cessation packages that may suit a variety of clinical settings.
Other extant research on cessation in the setting of LCS in the United States and abroad enriches the set of efficacious treatment models.21,22,23,24,25,26,27,28,29,30,31 Furthermore, building on the SCALE Collaboration, the US National Cancer Institute expects to fund up to three projects as part of the Scaling-up and Maintaining Evidence-based Interventions to Maximize Impact on Cancer (SUMMIT) Initiative. These projects will address the full process of LCS, including smoking cessation (RFA-CA-25-009).
More research will be needed to understand how best to incorporate cessation assistance into LCS. The SCALE Collaboration has provided evidence for several efficacious approaches for the integration of cessation at the point of LCS; other studies might build evidence about integrating cessation in shared decision-making prior to LCS referral or at other critical points in the clinical pathway.
References
- 1. US Preventive Services Task Force. Screening for Lung Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325(10):962-970. doi:10.1001/jama.2021.1117
- 2. G. Slatore C, Baumann C, Pappas M, Humphrey LL. Smoking Behaviors among Patients Receiving Computed Tomography for Lung Cancer Screening. Systematic Review in Support of the U.S. Preventive Services Task Force. Ann Am Thorac Soc. 2014;11(4):619-627. doi:10.1513/AnnalsATS.201312-460OC
- 3. Cadham CJ, Cao P, Jayasekera J, et al. Cost-Effectiveness of Smoking Cessation Interventions in the Lung Cancer Screening Setting: A Simulation Study. J Natl Cancer Inst. 2021;113(8):1065-1073. doi:10.1093/jnci/djab002
- 4. Meza R, Cao P, Jeon J, et al. Impact of Joint Lung Cancer Screening and Cessation Interventions Under the New Recommendations of the U.S. Preventive Services Task Force. J Thorac Oncol. 2022;17(1):160-166. doi:10.1016/j.jtho.2021.09.011
- 5. Joseph AM, Rothman AJ, Almirall D, et al. Lung Cancer Screening and Smoking Cessation Clinical Trials. SCALE (Smoking Cessation within the Context of Lung Cancer Screening) Collaboration. Am J Respir Crit Care Med. 2018;197(2):172-182. doi:10.1164/rccm.201705-0909CI
- 6. Lawrence L. Data Supports Smoking Cessation as Part Active Lung Cancer Treatment. 2022. https://www.ilcn.org/data-supports-smoking-cessation-as-part-active-lung-cancer-treatment/
- 7. National Cancer Institute. Smoking Cessation at Lung Examination: The SCALE Collaboration. 2024. https://cancercontrol.cancer.gov/brp/tcrb/scale-collaboration
- 8. Taylor KL, Williams RM, Li T, et al. A Randomized Trial of Telephone-Based Smoking Cessation Treatment in the Lung Cancer Screening Setting. J Natl Cancer Inst. 2022;114(10):1410-1419. doi:10.1093/jnci/djac127
- 9. Cao P, Smith L, Mandelblatt JS, et al. Cost-Effectiveness of a Telephone-Based Smoking Cessation Randomized Trial in the Lung Cancer Screening Setting. JNCI Cancer Spectr. 2022;6(4):pkac048. doi:10.1093/jncics/pkac048
- 10. Foley KL, Miller DP, Weaver K, et al. The OaSiS trial: A hybrid type II, national cluster randomized trial to implement smoking cessation during CT screening for lung cancer. Contemp Clin Trials. 2020;91:105963. doi:10.1016/j.cct.2020.105963
- 11. Foley KL, Dressler EV, Weaver KE, et al. The Optimizing Lung Screening Trial (WF-20817CD): Multicenter Randomized Effectiveness Implementation Trial to Increase Tobacco Use Cessation for Individuals Undergoing Lung Screening. Chest. 2023;164(2):531-543. doi:10.1016/j.chest.2023.03.013
- 12. Bellinger C, Foley KL, Dressler EV, et al. Organizational Characteristics and Smoking Cessation Support in Community-Based Lung Cancer Screening Programs. J Am Coll Radiol JACR. 2022;19(4):529-533. doi:10.1016/j.jacr.2022.01.014
- 13. Fu SS, Rothman AJ, Vock DM, et al. Optimizing Longitudinal Tobacco Cessation Treatment in Lung Cancer Screening: A Sequential, Multiple Assignment, Randomized Trial. JAMA Netw Open. 2023;6(8):e2329903. doi:10.1001/jamanetworkopen.2023.29903
- 14. Cinciripini PM, Minnix JA, Kypriotakis G, et al. Smoking Cessation Interventions in the Lung Cancer Screening Setting: A Randomized Clinical Trial. JAMA Intern Med. Published online January 13, 2025. doi:10.1001/jamainternmed.2024.7288
- 15. Park ER, Haas JS, Rigotti NA, et al. Integrating Tobacco Treatment Into Lung Cancer Screening: The Screen Assist Factorial Randomized Clinical Trial. JAMA Intern Med. Published online March 03, 2025. doi:10.1001/jamainternmed.2024.8399
- 16. Williams RM, Eyestone E, Smith L, et al. Engaging Patients in Smoking Cessation Treatment within the Lung Cancer Screening Setting: Lessons Learned from an NCI SCALE Trial. Curr Oncol. 2022;29(4):2211-2224. doi:10.3390/curroncol29040180
- 17. Lang AE. Update on the National Cancer Institute’s Smoking Cessation at Lung Examination Collaboration Trials. Chest. 2024;165(6):1302-1306. doi:10.1016/j.chest.2023.12.016
- 18. Zeliadt S. Best Practices for Implementation of Tobacco Treatment in Lung Cancer Screening: Lessons Learned from the SCALE Collaboration. Presented at: American Cancer Society National Lung Cancer Roundtable Annual Meeting; December 2024; Atlanta, GA.
- 19. Toll B. Best Practices for Implementation of Tobacco Treatment in Lung Cancer Screening: Lessons Learned from the SCALE Collaboration. Presented at: American Cancer Society National Lung Cancer Roundtable Annual Meeting; December 2024; Atlanta, GA.
- 20. Ostroff, J. Best Practices for Implementation of Tobacco Treatment in Lung Cancer Screening: Lessons Learned from the SCALE Collaboration. Presented at: American Cancer Society National Lung Cancer Roundtable Annual Meeting; December 2024; Atlanta, GA.
- 21. Kohn R, Vachani A, Small D, et al. Comparing Smoking Cessation Interventions among Underserved Patients Referred for Lung Cancer Screening: A Pragmatic Trial Protocol. Ann Am Thorac Soc. 2022;19(2):303-314. doi:10.1513/AnnalsATS.202104-499SD
- 22. Kim Y, Lee J, Lee E, et al. Strategies to Improve Smoking Cessation for Participants in Lung Cancer Screening Program: Analysis of Factors Associated with Smoking Cessation in Korean Lung Cancer Screening Project (K-LUCAS). Cancer Res Treat. 2024;56(1):92-103. doi:10.4143/crt.2022.1598
- 23. Williams PJ, Philip KE, Alghamdi SM, et al. Strategies to deliver smoking cessation interventions during targeted lung health screening – a systematic review and meta-analysis. Chron Respir Dis. 2023;20:14799731231183446. doi:10.1177/14799731231183446
- 24. Moldovanu D, de Koning HJ, van der Aalst CM. Lung cancer screening and smoking cessation efforts. Transl Lung Cancer Res. 2021;10(2):1099-1109. doi:10.21037/tlcr-20-899
- 25. Pastorino U, Ladisa V, Trussardo S, et al. Cytisine Therapy Improved Smoking Cessation in the Randomized Screening and Multiple Intervention on Lung Epidemics Lung Cancer Screening Trial. J Thorac Oncol Off Publ Int Assoc Study Lung Cancer. 2022;17(11):1276-1286. doi:10.1016/j.jtho.2022.07.007
- 26. Cartmel B, Fucito LM, Bold KW, et al. Effect of a Personalized Tobacco Treatment Intervention on Smoking Abstinence in Individuals Eligible for Lung Cancer Screening. J Thorac Oncol. 2024;19(4):643-649. doi:10.1016/j.jtho.2023.11.012
- 27. Murray RL, Brain K, Britton J, et al. Yorkshire Enhanced Stop Smoking (YESS) study: a protocol for a randomised controlled trial to evaluate the effect of adding a personalised smoking cessation intervention to a lung cancer screening programme. BMJ Open. 2020;10(9):e037086. doi:10.1136/bmjopen-2020-037086
- 28. Murray RL, Alexandris P, Baldwin D, et al. Uptake and 4-week quit rates from an opt-out co-located smoking cessation service delivered alongside community-based low-dose computed tomography screening within the Yorkshire Lung Screening Trial. Eur Respir J. 2024;63(4):2301768. doi:10.1183/13993003.01768-2023
- 29. NIH. NIH RePORT. [cited 2025 Jan 29]. Providing Tobacco Treatment to Patients Undergoing Lung Cancer Screening at MedStar Health: A Randomized Trial. Available from: https://reporter.nih.gov/search/puTxItU_70yawpTrT6TESQ/project-details/10809717#description
- 30. Williams PJ, Philip KEJ, Buttery SC, Perkins A, Chan L, Bartlett EC, et al. Immediate smoking cessation support during lung cancer screening: long-term outcomes from two randomised controlled trials. Thorax. 2024 Feb 15;79(3):269–73.
- 31. Iivanainen S, Kurtti A, Wichmann V, Andersen H, Jekunen A, Kaarteenaho R, et al. Smartphone application versus written material for smoking reduction and cessation in individuals undergoing low-dose computed tomography (LDCT) screening for lung cancer: a phase II open-label randomised controlled trial. The Lancet Regional Health – Europe [Internet]. 2024 Jul 1 [cited 2025 Jan 29];42. Available from: https://www.thelancet.com/journals/lanepe/article/PIIS2666-7762(24)00113-3/fulltext