The first-in-class KRAS G12C inhibitor sotorasib has demonstrated early, deep, and durable responses in the treatment of advanced NSCLC with KRAS G12C mutations, according to results from the registrational phase II CodeBreaK 100 trial.
“Breakthrough therapy designation was granted by U.S. Food and Drug Administration (FDA), and I am pleased to report that regulatory filings to the FDA and European Medicines Agency have been submitted,” said Bob T. Li, MD, of Memorial Sloan Kettering Cancer Center, during his Presidential Symposium presentation. “The confirmatory phase III part of the CodeBreaK 200 trial evaluating sotorasib versus second-line docetaxel is currently enrolling.”
KRAS is the most frequently mutated oncogene in human cancers, and despite its discovery nearly 40 years ago, there is no approved KRAS-targeting therapy. KRAS G12C mutation is a prevalent oncogenic driver responsible for approximately 13% of lung adenocarcinomas and is associated with poor patient outcomes, Dr. Li said.
In the phase I CodeBreaK 100 trial sotorasib showed durable clinical benefit in a cohort of 59 patients with heavily pretreated NSCLC. The objective response was 32.2%, with a median duration of response of 10.9 months. The median progression-free survival was 6.3 months.
The phase II trial included 126 patients with locally advanced or metastatic NSCLC with KRAS G12C mutations. Patients had progressed on no more than three prior standard therapies; 81% of included patients had progressed on prior platinum-based chemotherapy and PD-L1 inhibitors. The primary endpoint was overall response rate as assessed by blinded independent central review.
Sotorasib was administered at 960 mg once daily until disease progression.
Of the 126 patients included, two did not have measurable disease at baseline per central radiology review and were excluded from the response assessment, Dr. Li said. Of the 124 patients, the overall response rate (ORR) was 37.1% including three patients with confirmed response and 43 with partial response. The disease control rate was 80.6%.
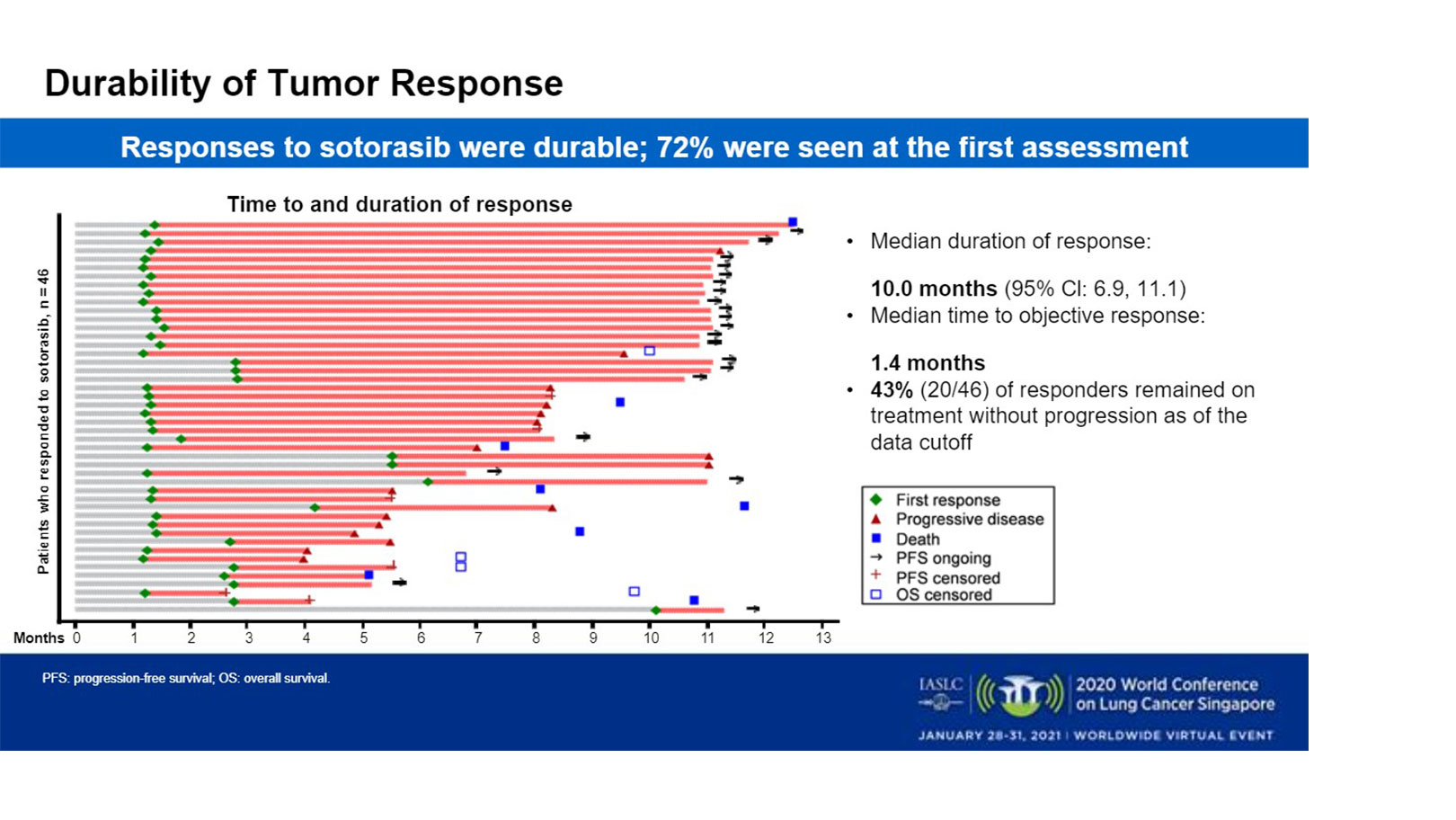
Tumor shrinkage of any magnitude was observed in 81% of patients. The median percentage of best tumor shrinkage among all responders was 60%. Responses were durable, Dr. Li said.
“Seventy-two percent of patients had an early rapid response on first CT scan at 6 weeks,” he said.
The median time to objective response was 1.4 months with a median duration of response of 10 months. Forty-three percent of responders remained on treatment without progression as of data cutoff.
The median progression-free survival was 6.8 months, which is consistent with the previous phase I results, Dr. Li said.
According to Dr. Li, sotorasib was well-tolerated, with no deaths attributed to treatment and a low incidence of treatment-related grade-3 or -4 adverse events, treatment discontinuation, and dose modification.
Treatment-related adverse events were generally mild and manageable; most were grade 1 or 2. There was one grade 4 toxicity in a patient who reported dyspnea and pneumonia.
Treatment-related adverse events led to dose modification in about one-fifth (22.2%) of patients and discontinuation in 7.1% of patients.
The researchers also conducted exploratory biomarker analyses using pretreatment tumor biopsies. Sotorasib responses were seen across subgroups. The overall response rate in PD-L1 TPS less than 1% was 48%; PD-L1 TPS 1% to 49%, overall response rate was 39%; and TPS greater than 50%, overall response rate was 22%.
The researchers also looked at comutation patterns associated with poor outcomes.
“We saw sotorasib responses across STK11 mutations, [as well as] KEAP1 mutants and wild-types,” Dr. Li said.
They also looked at other markers like TP53 mutations and saw responses across both mutant and wild-types.
Breaking New Ground
Commenting on the study, Pasi A. Jänne, MD, PhD, of Dana Farber Cancer Institute, congratulated the researchers and declared that KRAS G12C inhibitors are finally here.
To put the importance of these findings in perspective, Dr. Jänne pointed out that half of patients with KRAS mutations have KRAS G12C mutations and that this subset is a greater number of patients than those with ALK, ROS1, RET, and TRK 1/2/3 mutations combined.
“Thus, [identification of] therapies for patients with KRAS G12C mutations represents a significant potential advance for patients with lung adenocarcinoma,” Dr. Jänne said.
In addition to sotorasib, there are a number of other agents targeting KRAS G12C in clinical and preclinical development, including adagrasib. In a phase I/II study, adagrasib showed a 45% response rate in a population of 51 patients with duration of response and progression-free survival not yet known.
“The good news is the KRAS G12C inhibitors are highly selected for mutant KRAS and, as such, they are quite well tolerated,” Dr. Jänne said. “If you look across clinical trials most treatment-related adverse events are grade 1 or 2 toxicities.”
Importantly treatment-related adverse events leading to discontinuation are less than 10% in all the trials to date.
“In aggregate, the toxicity profile is similar to our other effective targeted therapies,” he said.
Some questions about these treatments remain though. First, why is the clinical activity of KRAS G12C inhibitors not greater?
Of the potential possibilities, one is that the activity is lower in pretreated patients. Another possibility is that KRAS G12C cancers are genomically more heterogeneous.
“These cancers occur more frequently in current or former smokers and are likely to be more complex genomically than EGFR mutant or ALK rearranged cancers,” Dr. Jänne said.
More work also needs to be done to identify if there are certain subsets of patients that benefit from KRAS G12C inhibitors, Dr. Jänne said.
“The first hint of this was from trials of adagrasib suggesting that those patients with concomitant STK11 mutation may have a higher response rate,” Dr. Jänne said. “We see a similar trend here for the sotorasib data presented by Dr. Li, with a 50% response rate in this population.”
Finally, Dr. Jänne pointed out that there are a lot of preclinical and clinical studies underway investigating various agents and combinations to target KRAS G12C.
“We look forward to the results of these studies in patients who have progressed on single-agent therapies or patients who have not responded to single-agent therapies,” he said.
This session had a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 90 days at wclc2020.iaslc.org.