Nivolumab significantly improved investigator-reported progression-free survival and overall survival compared with placebo in patients with relapsed malignant mesothelioma, according to preliminary results of the CONFIRM phase III trial presented during the Presidential Symposium.
“Nivolumab is a safe and effective treatment and should be considered a new treatment option for patients with relapsed mesothelioma,” said Dean Fennell, FRCP, PhD, of University of Leicester, United Kingdom, who presented the results.
In 2021, effective therapy for relapsed mesothelioma remains an unmet need with no randomized phase III trial demonstrating improved overall survival in the relapsed setting, Dr. Fennell said.
The CONFIRM trial was a Stand Up to Cancer–/Cancer Research UK–supported, investigator-initiated study designed to evaluate the efficacy of nivolumab. It was the first-ever placebo-controlled phase III trial of an anti–PD-1 immune checkpoint inhibitor in relapsed mesothelioma, according to Dr. Fennell.
In the study, patients were randomly assigned 2:1 to nivolumab 240 mg on a 14-day cycle (221 patients) or placebo (111 patients). The co-primary outcomes were overall survival and investigator-reported progression-free survival. The study included patients with mesothelioma who had been treated with at least one prior line of therapy.
The study was halted due to COVID-19 with 332 patients randomly assigned, Dr. Fennell noted. However, it was decided that there were sufficient events to allow the study to stop.
Baseline characteristics were well-balanced between the two treatment arms with slightly more patients with PD-L1 tumor proportion score (TPS) of 1% or greater in the nivolumab arm (37% vs. 29%).
The majority (95%) of disease in both arms was of pleural origin.
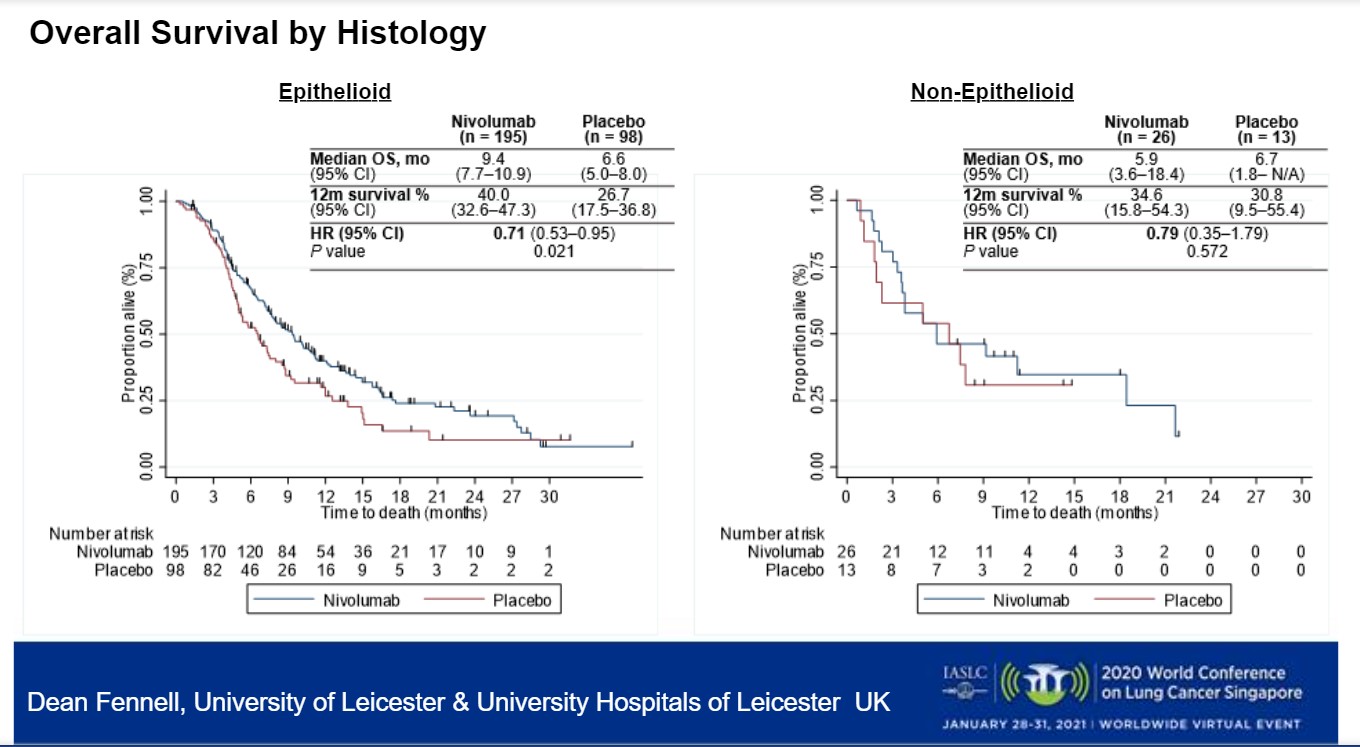
There was an overall survival benefit in favor of nivolumab (HR 0.72; 95% CI: 0.55, 0.94). The 12-month overall survival was 39.5% for nivolumab compared with 26.9% for placebo.
Progression-free survival also favored nivolumab (HR 0.61; 95% CI: 0.48, 0.77; p < 0.001). The median progression-free survival was 3.0 months compared with 1.8 months for placebo. The 12-month progression-free survival was 14.5% for nivolumab compared with 4.9% for placebo. Dr. Fennell noted that the survival results were not yet mature with around 80% of required target events.
“There was no evidence to support PD-L1 TPS as being predictive,” Dr. Fennell said.
However, significant clinical benefit was observed by subtype (Figure). Patients with epithelioid disease benefited from nivolumab with an HR for overall survival of 0.71 (95% CI: 0.53, 0.95; p = 0.021). However, the same was not true for non-epithelioid disease (HR 0.79; 95% CI: 0.35, 1.79; p = 0.572).
The interquartile range for duration of treatment was 84 days in the nivolumab arm compared with 43 days in the placebo arm. The number of participants receiving further immunotherapy was 1.4% in the nivolumab arm compared with 12.6% in the placebo arm.
The proportion of patients with greater than grade 3 toxicity was 45% for nivolumab compared with 42% for placebo; for serious adverse events the rates were 36% and 39%, respectively. The proportion of deaths due to serious adverse events was 3.6% for nivolumab and 5.3% for placebo.
Long-Awaited Advances
Commenting on the study, Rina Hui, MBBS, PhD, of Crown Princess Mary Cancer Centre, Westmead Hospital, The University of Sydney, Australia, called the positive phase III study of a checkpoint inhibitor in relapsed mesothelioma a “long time coming.”
The phase III CONFIRM study is a positive study having met both survival endpoints with a 12.6% improvement in 12-month survival, she said. However, there were no clear predictive biomarkers for patient selection.
Prior to this, there have been limited data on salvage treatment for relapsed mesothelioma, Dr. Hui said.
Other than CONFIRM, the only phase III trial of immunotherapy in relapsed mesothelioma—PROMISE1— showed no benefit to checkpoint inhibition pembrolizumab compared with single-agent chemotherapy in the second-line setting in terms of progression-free or overall survival, Dr. Hui said.
She also pointed out strengths of CONFIRM in recruiting 332 patients, a large proportion of which were heavily pretreated. Although CONFIRM did not find PD-L1 to be predictive of benefit, Dr. Hui was not surprised because PD-L1 as a predictive biomarker in mesothelioma has been controversial.
“Some phase II studies showed improved outcomes in patients with PD-L1–positive disease while some did not,” Dr. Hui said, noting that the proportion of PD-L1–positive disease in the CONFIRM study was much lower than in prior studies, and only 70% of the tumors were evaluable for PD-L1 status.
Addressing the histologic results, Dr. Hui said that non-epithelioid subtype is known to be the more aggressive, chemo-resistant subtype as indicated by the steep decline in the survival curve in the chemotherapy arm of the first-line CheckMate 743 study2 that compared nivolumab plus ipilimumab to chemotherapy.
“A lot of patients would not have made it to a subsequent line clinical trial after chemotherapy, explaining why this subtype was only 12% of the CONFIRM study,” Dr. Hui said. “The first-line trial showed an increased benefit with nivolumab plus ipilimumab in non-epithelioid subtype likely due to association with high level of PD-L1. The sample size of the non-epithelioid subgroup in CONFIRM may have been too small to detect a difference in outcomes.”
Despite this, Dr. Hui said that she “would not deny patients with non-epithelioid histology from considering nivolumab in the salvage setting.”
Moving forward, much still needs to be learned, Dr. Hui said. Because this study was placebo controlled, questions remain about whether nivolumab provides better outcomes than second-line chemotherapy or second-line gemcitabine plus VEGF inhibitor ramucirumab. There are also questions about what salvage therapy to use if nivolumab plus ipilimumab is used in the first-line setting.
“We need to identify predictive biomarkers, and we eagerly await results from other first-line immunotherapy combination studies,” Dr. Hui said.
This session had a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 90 days at wclc2020.iaslc.org.
References:
- Popat S, Curioni-Fontecedro A, Dafni U, et al. A multicentre randomized phase III trial comparing pembrolizumab versus single-agent chemotherapy for advanced pre-treated malignant pleural mesothelioma: the European Thoracic Oncology Platform (ETOP 9-15) PROMISE-meso trial. Ann Oncol. 2020;31(12):1734-1745.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636-2644.