Patients with metastatic NSCLC with a PD-L1 tumor proportion score (TPS) ≥ 50% face greater toxicity without the benefit of enhanced efficacy when ipilimumab is combined with pembrolizumab in the first-line setting, according to findings of the phase III KEYNOTE-598 trial. In fact, the results at the first interim analysis for the immunotherapy combination were so underwhelming that the external data monitoring committee recommended stopping ipilimumab and placebo administration due to futility.
“As a result, pembrolizumab monotherapy remains a standard-of-care first-line treatment for NSCLC with TPS of at least 50% and no targetable EGFR or ALK aberrations. The results of this study will be published simultaneously in the Journal of Clinical Oncology,” stated Michael Boyer, MD, a clinical professor of medicine at the Chris O’Brien Lifehouse and the Central Clinical School of the University of Sydney, who presented the KEYNOTE-598 findings during the Presidential Symposium.
KEYNOTE-024 previously established pembrolizumab monotherapy as a new standard of care for patients with metastatic NSCLC featuring a PD-L1 TPS ≥ 50% and no targetable EGFR or ALK aberrations based on a significant overall survival (OS) advantage over platinum-doublet chemotherapy.1 Although median OS improved from 13.4 months with chemotherapy to 26.3 months with pembrolizumab, the fact that 20% of pembrolizumab-treated patients had died after 6 months of treatment still left marked room for improvement.1,2
Dr. Boyer explained that although agents targeting PD-1 and CTLA-4 are all considered immune checkpoint inhibitors, these agents elicit different immune effects based on their distinct targets. For this reason, attempts have been made to combine these drugs to leverage their different and potentially synergistic mechanisms of action to improve patient outcomes—and with success. Dual immunotherapy with nivolumab and ipilimumab constitutes a standard of care in other advanced malignancies such melanoma and renal cell carcinoma, and results from CheckMate 227 recently showed that first-line treatment with nivolumab plus ipilimumab significantly prolongs OS in comparison with chemotherapy in patients with NSCLC featuring PD-L1 expression of ≥ 1%.3
Despite these results, “to date, there are no appropriately powered or controlled comparisons of anti–PD-1 monotherapy versus dual anti–PD-1 and anti–CTLA-4 therapy as first-line treatment for NSCLC,” Dr. Boyer stated.
KEYNOTE-598 was designed to address this data void in stage IV NSCLC, specifically in disease with a PD-L1 TPS ≥ 50%. The global trial included 568 patients who had no sensitizing EGFR mutations or known ALK alterations and who had not received prior systemic therapy. Following stratification by European Cooperative Oncology Group performance status (0 vs 1), region (East Asia vs not East Asia), and histology (squamous vs nonsquamous), patients underwent random assignment in a double-blind fashion to pembrolizumab plus ipilimumab or pembrolizumab plus placebo. Pembrolizumab 200 mg was administered to patients in both arms every 3 weeks for up to 35 cycles, while ipilimumab 1 mg/kg or saline placebo was administered every 6 weeks for up to 18 cycles.
At the first interim analysis, OS, one of the co-primary endpoints along with progression-free survival (PFS), was examined for futility.
Based on a median follow-up of 20.6 months, median OS reached 21.4 months for patients treated with pembrolizumab/ipilimumab compared with 21.9 months for patients treated with pembrolizumab/placebo (HR, 1.08; 95% CI: 0.85-1.37; P = 0.74). The difference between arms for restricted mean survival time was -0.56 at the maximum observation time and -0.52 at 24 months, both meeting the futility criteria (ie, ≤ 0.2 at the maximum observation time and ≤ 0.1 at 24 months). None of the subgroups evaluated showed a survival benefit for pembrolizumab/ipilimumab over pembrolizumab/placebo.
Other efficacy outcomes showed nearly no difference between arms. Median PFS by blinded independent central review reached 8.2 months with ipilimumab/pembrolizumab compared with 8.4 months for placebo/pembrolizumab (HR, 1.06; 95% CI: 0.86-1.30; P = 0.72). The objective response rate was 45.4% in both arms. Among patients who responded to treatment, the median duration of response was 16.1 months with ipilimumab/pembrolizumab versus 17.3 months with placebo/pembrolizumab.
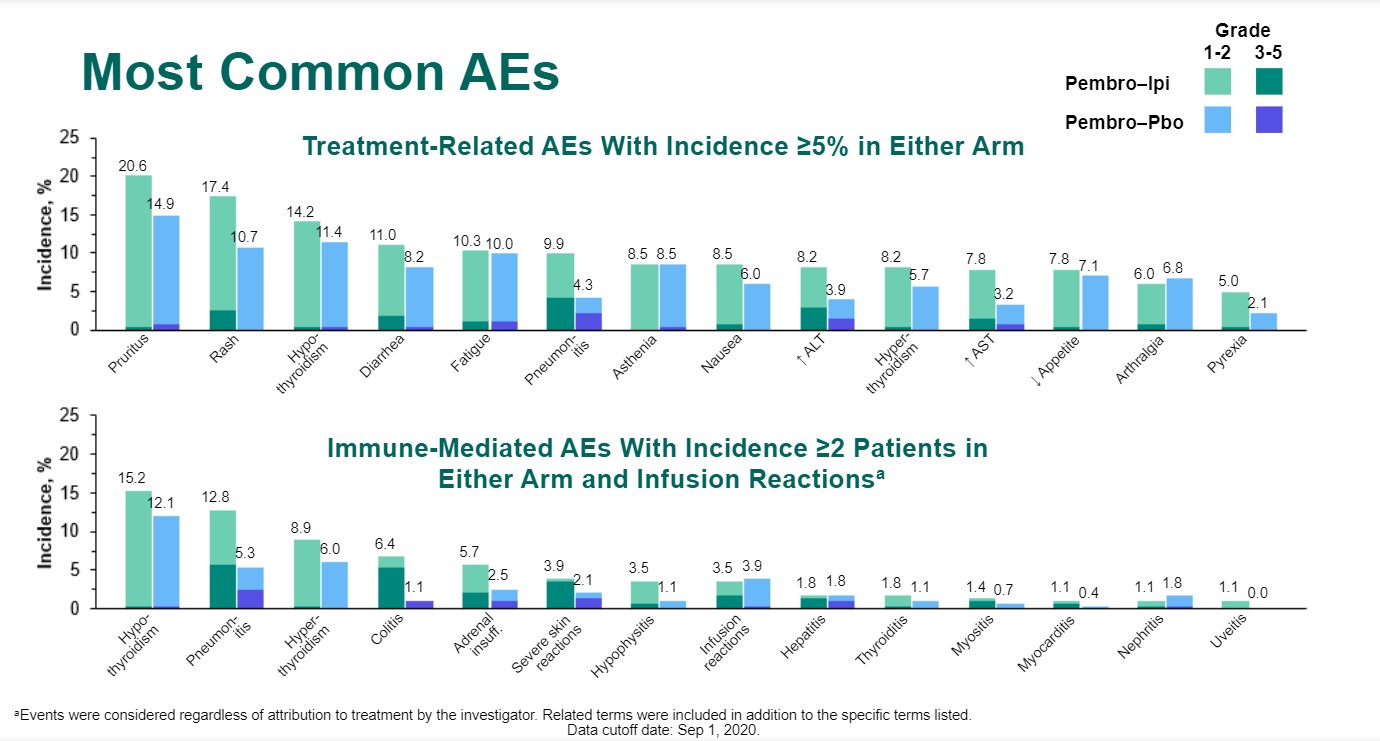
Treatment-related adverse events (AEs) were consistently more frequent with use of ipilimumab/pembrolizumab versus pembrolizumab alone, whether it be all-grade AEs (76.2% vs 68.3%), grade 3-5 AEs (35.1% vs 19.6%), AEs leading to death (2.5% vs 0%), or the need to discontinue any treatment (25.2% vs 10.7%). The same held true for immune-mediated AEs and infusion reactions, be it all-grade AEs (44.7% vs 32.4%), grade 3-5 AEs (20.2% vs 7.8%), those leading to death (2.1% vs 0%), and those necessitating discontinuation of any treatment (14.9% vs 5.3%).
Discussion
Discussant Fan Yun, MD, the Director of Thoracic Oncology at the Cancer Hospital of the University of Chinese Academy of Sciences (Zhejiang Cancer Hospital), noted that the OS and PFS outcomes observed with ipilimumab plus pembrolizumab in KEYNOTE-598 were quite comparable to those reported for ipilimumab plus nivolumab in CheckMate 227 in patients with PD-L1 tumor expression ≥ 50%.2 Likewise, the efficacy of pembrolizumab monotherapy was similar across the KEYNOTE-598 and KEYNOTE-024 trials.1
“All efficacy data in the KEYNOTE-598 study are consistent with relevant results of other studies,” Dr. Yun emphasized.
To account for the negative KEYNOTE-598 findings, Dr. Yun proposed that dual immunotherapy may not work in patients with ≥ 50% tumor PD-L1 expression because the conditions for immune checkpoint blockade are already optimized.
“As we know, CTLA-4 blockade allows for activation and proliferation of extra T-cell clones. However, the PD-L1 ≥ 50% population presents with a high level of preactivated CD8+ T cells in the immune microenvironment. Hence, additional CTLA-4 blockade may not bring clinical benefits, as expected,” Dr. Yun explained.
The same does not appear to hold true for other patient populations. Dr. Yun pointed to other data suggesting that dual immune checkpoint blockade offers benefit to patients with a PD-L1 TPS < 1% and those with high tumor mutation burden.
Although the ipilimumab-pembrolizumab combination failed in patients with advanced NSCLC with a PD-L1 TPS ≥ 50%, other immunotherapy combinations may yet show promise in this population, according to Dr. Yun. These combinatorial approaches include atezolizumab plus tiragolumab, the bifunctional fusion protein M7824, and pembrolizumab plus lenvatinib, all of which are currently being evaluated in phase II and III clinical trials.
References
- Reck M, Rodríguez-Abreu D, Robinson AG, et al; KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
- Brahmer JR, Rodriguez-Abreu D, Robinson AG, et al. KEYNOTE-024 5-year OS update: first-line (1L) pembrolizumab (pembro) vs platinum-based chemotherapy (chemo) in patients (pts) with metastatic NSCLC and PD-L1 tumour proportion score (TPS) ≥50%. Ann Oncol. 2020;31(suppl 4):S1142-S1215.
- Hellmann MD, Paz-Ares L, Bernabe Caro R, et al. Nivolumab plus ipilimumab in advanced non-small-cell lung cancer. N Engl J Med. 2019;381(21):2020-2031.