Spine metastases represent a common complication of lung cancer and other cancers, with approximately 40% of patients developing clinically significant spinal metastatic disease and up to 90% developing subclinical micrometastases. In addition to site-associated pain, spine metastases can also cause neurologic deficits, reduced mobility, and a decline in a patient’s performance status and quality of life. Time-consuming efforts focused at managing these issues may delay overall cancer-directed therapy.
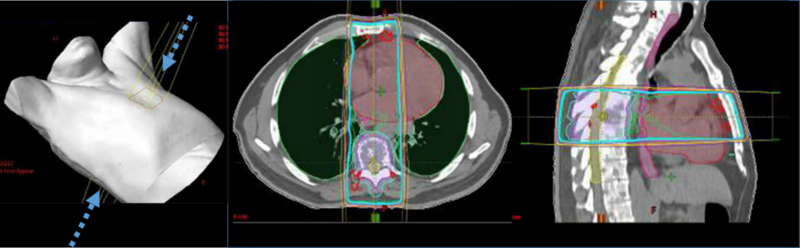
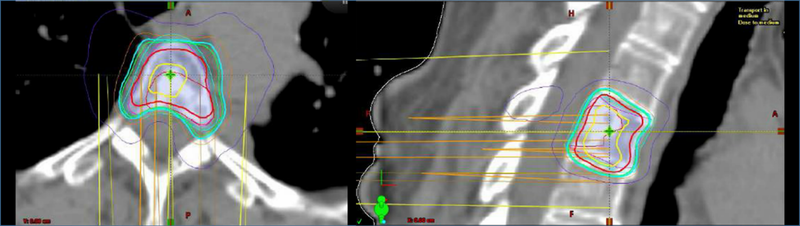
Traditional conventional radiotherapy techniques (either 2D or 3D conformal radiotherapy) have been used in patients with osseous metastatic disease, including spine metastases (Fig. 1). Stereotactic radiosurgery (SRS) techniques, initially developed for patients with intracranial metastasis, have increasingly been tested in patients with spine metastasis with encouraging results (Fig. 2).
Figure 1
In general, there are differences in at least four key outcome areas when spine SRS is compared with conventional palliative radiotherapy: overall pain response, durability of pain relief, tumor control (including those with radioresistant disease), and neurologic deficit improvement. First, although several conventional palliative fractionation schedules have been evaluated for patients with osseous metastasis, including 8 Gy in 1 fraction, 20 Gy in 5 fractions, and 30 Gy in 10 fractions, the rates of overall pain control remain modest. The RTOG 97-14 trial randomly assigned 897 patients to either 8 Gy in 1 fraction or to 30 Gy in 10 fractions.1
Overall, there was no difference in complete or partial pain relief (65% vs. 66%). In the subset of patients with spine metastasis, only 61% had complete or partial pain relief 1 month post-treatment. On the other hand, in a series of 500 patients treated with spine SRS, long-term pain improvement was observed in 86% of cases; obviously, it must be acknowledged that there were technology-imposed differences between these two patient cohorts.2
Figure 2
Second, durability of pain relief after treatment with conventional palliative radiotherapy wanes with time, especially 6 to 12 months after treatment.1 A prospective phase I/II trial of spine SRS in 149 patients with treatment of 166 lesions demonstrated not only enhanced durability of pain control, but also continuous improvement over time.3
Third, although tumor control rates are modest with conventional palliative radiotherapy fractionation schedules,4
local control rates are very high with spine SRS regardless of fractionation schedule.5
Moreover, although select tumor types that are often termed “radioresistant” (e.g., sarcoma, melanoma, gastrointestinal, and renal cell carcinoma) do not respond well to conventional low-dose palliation,6
studies evaluating spine SRS have demonstrated impressive response rates, regardless of primary tumor histology.7
Fourth, multiple series have demonstrated only modest improvements in tumor-associated neurologic deficits with low-dose conventional palliative regimens across radiosensitive and radioresistant histologies.6
In contrast, spine SRS has been used in patients with epidural disease to decompress the extraosseous disease extension and improve neurologic function.8
In one series of 62 patients receiving treatment to 85 spine lesions causing epidural compression, the mean epidural volume reduction was 65%, with associated improvements in thecal sac patency and neurologic function. Together, these studies have clearly suggested the putative value of spine SRS to provide durable local disease control, sustained pain relief, and neurologic stabilization or improvement.
Despite these promising institutional series, multi-institutional studies, and meta-analyses, most of which included patients with lung cancer, randomized trials of similar populations have not consistently replicated these results. RTOG 0631 randomly assigned patients with localized spine metastases to receive spine SRS (16 or 18 Gy in 1 fraction) or conventional palliative radiotherapy (8 Gy in 1 fraction).9
The primary endpoint for the phase III component—pain control at the treated site 3 months post-treatment—was no different between the two treatment arms (40.3% vs. 57.9%, p = 0.99), and there were no significant differences between the arms with respect to adverse events or quality-of-life parameters. A smaller phase II trial randomly assigned patients with localized spine metastases to spine SRS (24 Gy in 1 fraction) or to conventional palliative radiotherapy (30 Gy in 10 fractions) with the primary endpoint of pain relief of more than 2 points using the visual analog scale 3 months following treatment.10
Although the primary endpoint for this trial was not met, there was a trend toward improved complete pain response rates in the spine SRS arm (43.5% vs. 17.4%, p = 0.057). In addition, pain decreased more quickly in the spine SRS arm and lower pain levels were reported at the 6-month follow-up assessments. Finally, in non-spine osseous metastasis, a randomized phase II trial demonstrated higher rates of pain relief (including complete and partial pain response) with single-fraction SRS (12 Gy for ≥4-cm lesions or 16 Gy for <4-cm lesions) compared with conventional palliative radiotherapy (30 Gy in 10 fractions).11
Together, these early randomized spine and non-spine metastasis studies have provided low-level evidence that SRS might improve pain outcomes, but careful patient and endpoint selection is critical in any randomized trial setting.
Recently, a phase II/III trial randomly assigned patients who had spine metastasis with a spinal instability neoplasia score (SINS) of less than 12 to spine SRS (24 Gy in 2 fractions) or conventional palliative radiotherapy (20 Gy in 5 fractions); the primary endpoint was complete pain response at 3 months post-treatment.12
At a median follow-up of 6.7 months, the complete response rate at 3 months was higher with SRS (35% vs. 14%, p = 0.0002), and this benefit persisted at 6 months. Furthermore, overall response rates (complete and partial response) were numerically higher at 3 and 6 months, and spine SRS yielded a significant improvement in pain response on multivariable analysis at both time points. Additionally, there was no difference in grade 3-4 adverse events between arms, including the rates of compression fracture. Interestingly, despite the improvement in pain response, local control, quality of life, and analgesic consumption were similar between the two arms.
Arjun Sahgal, MD, and colleagues have advanced our understanding of spine SRS with this trial, and the strategy is clearly important for the field of spine oncology. Yet, additional analyses warrant consideration. Will the pain-relief curves continue to differ on long-term follow-up? Would pain reported as an “area under the curve,” capturing the patient’s entire pain journey, be more meaningful than a single-time landmark analysis? Additionally, although the study was not powered for radiation site-specific PFS rates, these were no different at 69% in patients who received conventional radiotherapy versus 75% in those who received spine SRS (p = 0.34). These additional observations invite critical introspection about the overall clinical benefit from such an approach. Could other variables explain the discrepancies across the studies performed to date? These and other questions need to be addressed upon long-term analysis of this trial, full publication of RTOG 0631, and the completion of similar studies.
In summary, SRS given at 24 Gy in 2 fractions was demonstrated to yield superior 3-month complete local-site pain relief compared to 20 Gy in 5 fractions. Should this be the current standard of care in clinical practice? The answer is yes, when it is available. This treatment is effective, it is associated with few side effects in well-selected patients, and it can be delivered over a short period requiring less time away from systemic therapy. However, the quality-of-life and medication-usage data do provide reason to pose the “net clinical benefit” question; hence, this strategy requires additional research. To this end, additional trials are underway to study alternative fractionation schedules (e.g., single-fraction vs. two-fraction SRS [NCT04218617] or dose-escalated hypofractionated techniques [NCT04802603]).
In the meantime, we believe strongly that even these positive results fall short of more meaningful outcomes. As a community of multidisciplinary oncologists, we cannot be satisfied that only a third of patients achieve complete local-site pain relief at 3 months with the latest radiotherapy techniques; we need to explore strategies to radically improve upon this. Adjusting fractionation schedules alone may be insufficient. Therefore, additional approaches to improve pain response (e.g., combining thermal ablation [NCT02713269] or radiofrequency ablation with radiotherapy [NCT04375891]) are under investigation.
- 1. Hartsell WF, Scott CB, Bruner DW, et al. Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst. 2005;97(11):798-804.
- 2. Gerszten PC, Burton SA, Ozhasoglu C, Welch WC. Radiosurgery for spinal metastases: clinical experience in 500 cases from a single institution. Spine (Phila Pa 1976). 2007;32(2):193-199.
- 3. Wang XS, Rhines LD, Shiu AS, et al. Stereotactic body radiation therapy for management of spinal metastases in patients without spinal cord compression: a phase 1-2 trial. Lancet Oncol. 2012;13(4):395-402.
- 4. Al-Omair A, Masucci L, Masson-Cote L, et al. Surgical resection of epidural disease improves local control following postoperative spine stereotactic body radiotherapy. Neuro Oncol. 2013;15(10):1413-1419.
- 5. Husain ZA, Sahgal A, De Salles A, et al. Stereotactic body radiotherapy for de novo spinal metastases: systematic review. J Neurosurg Spine. 2017;27(3):295-302.
- 6. a. b. Gerszten PC, Mendel E, Yamada Y. Radiotherapy and radiosurgery for metastatic spine disease: what are the options, indications, and outcomes? Spine (Phila Pa 1976). 2009;34(22 Suppl):S78-92.
- 7. Yamada Y, Katsoulakis E, Laufer I, et al. The impact of histology and delivered dose on local control of spinal metastases treated with stereotactic radiosurgery. Neurosurg Focus. 2017;42(1):E6.
- 8. Ryu S, Rock J, Jain R, et al. Radiosurgical decompression of metastatic epidural compression. Cancer. 2010;116(9):2250-2257.
- 9. Ryu S, Deshmukh S, Timmerman RD, et al. Radiosurgery Compared To External Beam Radiotherapy for Localized Spine Metastasis: Phase III Results of NRG Oncology/RTOG 0631. Int J Radiat Oncol Biol Phys. 2019;105(1):S2-S3.
- 10. Sprave T, Verma V, Forster R, et al. Randomized phase II trial evaluating pain response in patients with spinal metastases following stereotactic body radiotherapy versus three-dimensional conformal radiotherapy. Radiother Oncol. 2018;128(2):274-282.
- 11. Nguyen QN, Chun SG, Chow E, et al. Single-Fraction Stereotactic vs. Conventional Multifraction Radiotherapy for Pain Relief in Patients With Predominantly Nonspine Bone Metastases: A Randomized Phase 2 Trial. JAMA Oncol. 2019;5(6):872-878.
- 12. Sahgal A, Myrehaug SD, Siva S, et al. Stereotactic body radiotherapy versus conventional external beam radiotherapy in patients with painful spinal metastases: an open-label, multicentre, randomised, controlled, phase 2/3 trial. Lancet Oncol. 2021;22(7):1023-1033.