Immune checkpoint inhibitors (ICIs) and molecular targeting drugs used against oncogenic drivers, such as EGFR or ALK, have dramatically changed the treatment of NSCLC.1-6 However, treatment options after those drugs are still limited, and cytotoxic anticancer drugs are the mainstay of subsequent treatment. In addition, because platinum-based combination therapy is the most successful combination with an ICI, platinum-based combination induction therapy and subsequent maintenance therapy are still widely used.7-9
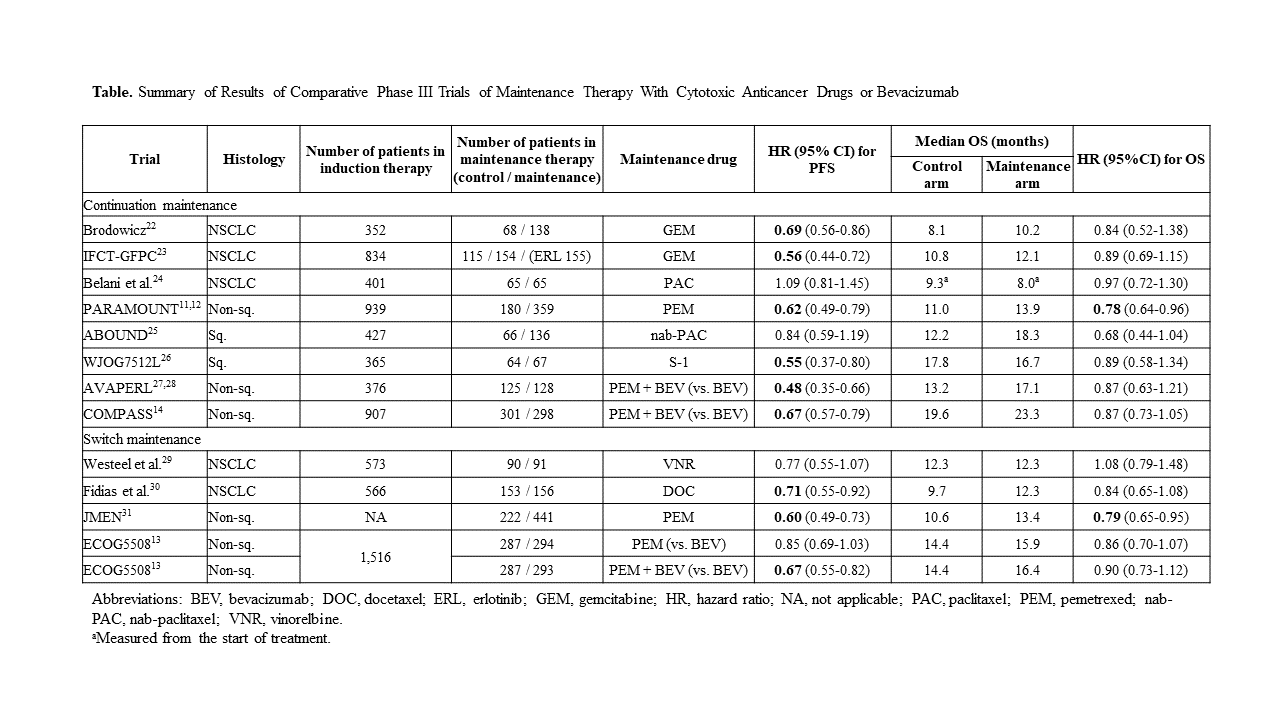
To date, various maintenance therapies have been developed to maximize the benefits of each drug (Table). Among them, standard combination therapy for nonsquamous NSCLC includes combination carboplatin/paclitaxel with bevacizumab and a platinum-based agent/pemetrexed combination, which are followed by maintenance therapy with bevacizumab alone or pemetrexed alone, respectively.10-12 In 2019, two important clinical trials investigating new maintenance therapies, ECOG-ACRIN 550813 and COMPASS (WJOG5610L),14 were reported.
Overall Survival Comparisons for Maintenance Therapies
The ECOG-ACRIN 5508 trial investigated OS after switching maintenance to either pemetrexed alone or pemetrexed plus bevacizumab versus continuous bevacizumab monotherapy after induction therapy with carboplatin and paclitaxel plus bevacizumab.13 Of the 1,516 patients enrolled, 874 (57%) were randomly assigned to bevacizumab, pemetrexed, or combination pemetrexed/bevacizumab maintenance therapy groups. The median OS was 14.4, 15.9 (HR 0.86; p = 0.12), and 16.4 (HR 0.9; p = 0.28) months for the three groups, respectively. The superiority of pemetrexed or combination pemetrexed /bevacizumab in OS was not shown.
The COMPASS trial examined OS after treatment with combination pemetrexed/bevacizumab versus bevacizumab alone as maintenance therapy for patients who received induction therapy with combination carboplatin/pemetrexed/bevacizumab.14 Of the 907 patients who received induction therapy, 599 were randomly assigned 1:1 to the bevacizumab-alone or pemetrexed/bevacizumab group. The median OS was 19.6 and 23.3 months (HR 0.87; p = 0.069), respectively. The superiority of pemetrexed/bevacizumab over bevacizumab monotherapy was not confirmed. However, it was suggested that patients without an EGFR mutation would benefit from maintenance therapy with pemetrexed/bevacizumab (OS HR 0.82; p = 0.020) and that pemetrexed would be essential for maintenance therapy when carboplatin/pemetrexed/bevacizumab is administered as induction therapy.
Maintenance therapy for nonsquamous NSCLC is still an indispensable part of cancer management. On the basis of the above results, we must decide which induction therapy and which maintenance therapy should be administered to maximize patient benefits.
The Era of Immune Checkpoint Inhibitors
For EGFR- and ALK-positive cases, the therapeutic effect of an ICI alone is limited.15-17 Regarding the combination of an ICI and chemotherapy, favorable outcomes of the combination of carboplatin/paclitaxel/bevacizumab with atezolizumab have been reported in patients with EGFR- and ALK-positive disease; however, this outcome is only from one report of an unplanned subgroup analysis.7,18 In addition, other ICI plus chemotherapy combinations either failed to show any additional effects in patients with EGFR and ALK alterations or excluded those with EGFR- and ALK-positive disease from the trials.8,9,19 Therefore, induction platinum-based combination therapy followed by maintenance chemotherapy is still an important treatment option for patients with driver oncogene–positive disease.
In patients who are positive for PD-L1, ICI alone or a combination of ICI and chemotherapy is the treatment option for initial treatment. If single-agent ICI is selected as the first-line treatment, platinum-based combination chemotherapy is still an important treatment option for the subsequent second-line treatment.
In addition, platinum-based induction chemotherapy and maintenance therapy is important as a first-line treatment for patients with contraindications for ICIs, such as autoimmune disease and interstitial pneumonia.
In this era of immunotherapy, the combination of an ICI with chemotherapy is frequently used as an initial treatment. If the induction therapy is a pemetrexed-based or bevacizumab-based regimen with an ICI, then pemetrexed plus an ICI or bevacizumab plus an ICI is selected as the maintenance therapy, respectively. In that sense, maintenance therapy with pemetrexed or bevacizumab continues to be used as an integral part of combination therapy with an ICI and chemotherapy.
Preclinically, it has been suggested that pemetrexed induces cancer immunity through immunogenic cell death.20 On the other hand, it has been suggested that bevacizumab, an anti-vascular endothelial growth factor antibody, alters the cancer microenvironment and tumor vessels to induce cancer immunity.21 Therefore, it is expected that there will be beneficial effects of using an ICI in combination with these drugs in not only the induction phase but also in the maintenance phase.
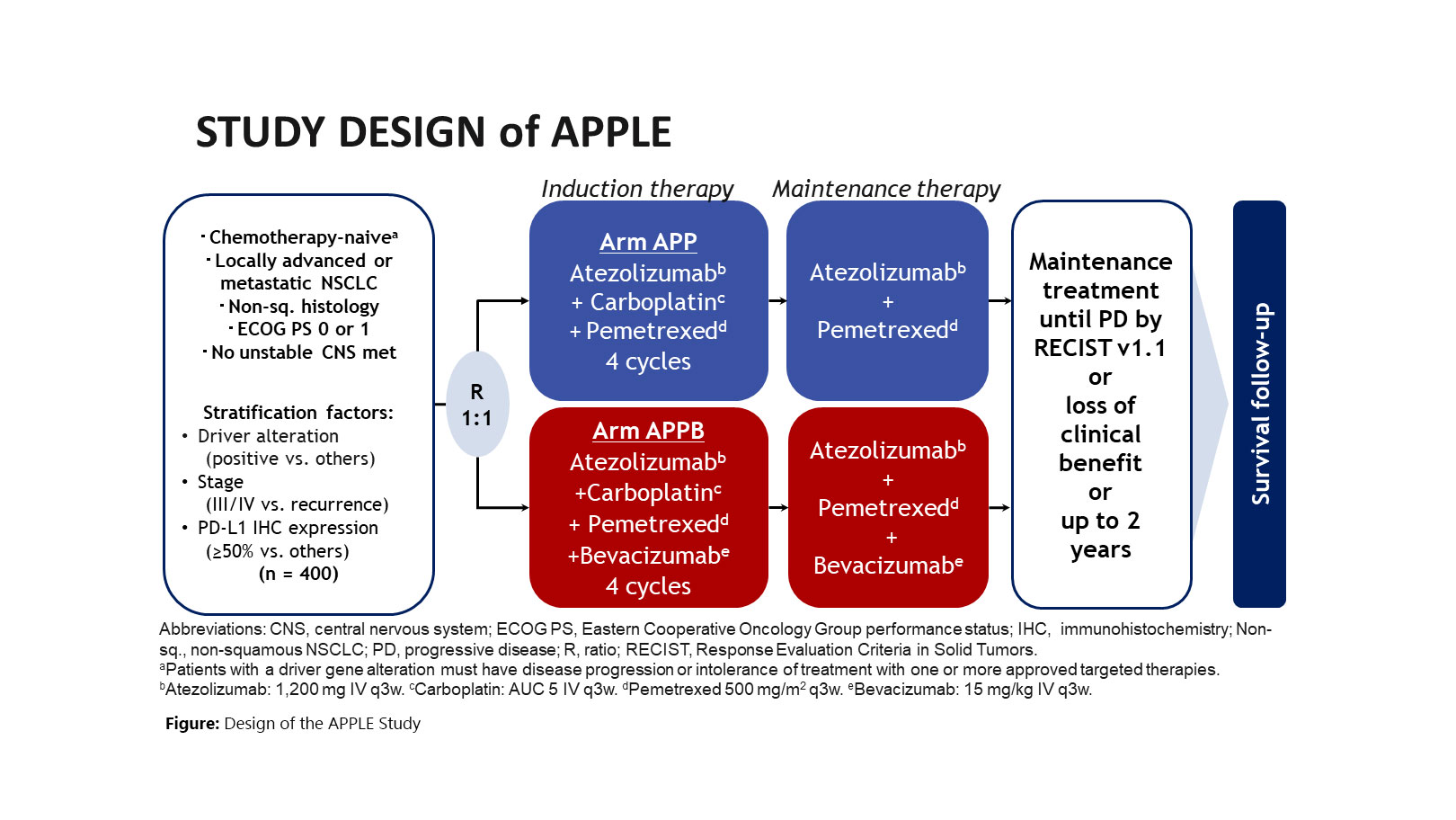
We are currently conducting the APPLE study based on the experience of treatment development in the COMPASS study (Fig.). The purpose of this study is to evaluate the effects of adding bevacizumab to carboplatin/pemetrexed/atezolizumab combination therapy. We will promote the development of treatments that further enhance the effects of ICIs by combining pemetrexed and bevacizumab with ICIs.
References:
- Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121-128.
- Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in untreated EGFR-mutated advanced non-small-cell lung cancer. N Engl J Med. 2018;378(2):113-125.
- Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. 2014;371(23):2167-2177.
- Hida T, Nokihara H, Kondo M, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet. 2017;390(10089):29-39.
- Reck M, Rodríguez-Abreu D, Robinson AG, et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823-1833.
- Mok TSK, Wu YL, Kudaba I, et al. Pembrolizumab versus chemotherapy for previously untreated, PD-L1-expressing, locally advanced or metastatic non-small-cell lung cancer (KEYNOTE-042): a randomised, open-label, controlled, phase 3 trial. Lancet. 2019;393(10183):1819-1830.
- Socinski MA, Jotte RM, Cappuzzo F, et al. Atezolizumab for first-line treatment of metastatic nonsquamous NSCLC. N Engl J Med. 2018;378(24):2288-2301.
- Gandhi L, Rodriguez-Abreu D, Gadgeel S, et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078-2092.
- Papadimitrakopoulou VA, Cobo M, Bordoni R, et al. IMpower132: PFS and safety results with 1L atezolizumab plus carboplatin/cisplatin plus pemetrexed in stage IV non-squamous NSCLC. Abstract presented at: 19th World Conference on Lung Cancer; 2018; Toronto, Canada. Abstract 12389.
- Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355(24):2542-2550.
- Paz-Ares L, de Marinis F, Dediu M, et al. Maintenance therapy with pemetrexed plus best supportive care versus placebo plus best supportive care after induction therapy with pemetrexed plus cisplatin for advanced non-squamous non-small-cell lung cancer (PARAMOUNT): a double-blind, phase 3, randomised controlled trial. Lancet Oncol. 2012;13(3):247-255.
- Paz-Ares LG, de Marinis F, Dediu M, et al. PARAMOUNT: final overall survival results of the phase III study of maintenance pemetrexed versus placebo immediately after induction treatment with pemetrexed plus cisplatin for advanced nonsquamous non-small-cell lung cancer. J Clin Oncol. 2013;31(23):2895-2902.
- Ramalingam SS, Dahlberg SE, Belani CP, et al. Pemetrexed, bevacizumab, or the combination as maintenance therapy for advanced nonsquamous non-small-cell lung cancer: ECOG-ACRIN 5508. J Clin Oncol. 2019;37(26):2360-2367.
- Seto T, Azuma K, Yamanaka T, et al. Randomized phase III study of continuation maintenance bevacizumab with or without pemetrexed in advanced nonsquamous non-small-cell lung cancer: COMPASS (WJOG5610L). J Clin Oncol. Published online December 27, 2019.
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639.
- Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387(10027):1540-1550.
- Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389(10066):255-265.
- Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387-401.
- West H, McCleod M, Hussein M, et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol. 2019;20(7):924-937.
- David AS, Sandaruwan G, Nelusha A, et al. The folate pathway inhibitor pemetrexed pleiotropically enhances effects of cancer immunotherapy. Clin Cancer Res. 2019;25(23):7175-7188.
- Ott PA, Hodi FS, Buchbinder EI. Inhibition of immune checkpoints and vascular endothelial growth factor as combination therapy for metastatic melanoma: an overview of rationale, preclinical evidence, and initial clinical data. Front Oncol. 2015;5:202.
- Brodowicz T, Krzakowski M, Zwitter M, et al. Cisplatin and gemcitabine first-line chemotherapy followed by maintenance gemcitabine or best supportive care in advanced non-small cell lung cancer: a phase III trial. Lung Cancer. 2006;52(2):155-163.
- Pérol M, Chouaid C, Pérol D, et al. Randomized, phase III study of gemcitabine or erlotinib maintenance therapy versus observation, with predefined second-line treatment, after cisplatin-gemcitabine induction chemotherapy in advanced non-small-cell lung cancer. J Clin Oncol. 2012;30(28):3516-3524.
- Belani CP, Barstis J, Perry M, et al. Multicenter, randomized trial for stage IIIB or IV non-small-cell lung cancer using weekly paclitaxel and carboplatin followed by maintenance weekly paclitaxel or observation. J Clin Oncol. 2003;21(15):2933-2939.
- Spigel DR, Jotte RM, Ponce Aix S, et al. Nab-paclitaxel plus carboplatin induction followed by nab-paclitaxel maintenance in squamous non-small cell lung cancer (NSCLC): results from the ABOUND.sqm study. Poster presented at: European Society for Medical Oncology; 2018; Munich, Germany.
- Kaoru T, Satoshi M, Masahiko Ando, et al. Ph3 study of maintenance therapy with S-1 vs BSC after induction therapy with carboplatin plus S-1 for advanced squamous cell lung cancer (WJOG7512L). Paper presented at: IASLC World Conference on Lung Cancer; 2019; Barcelona, Spain.
- Barlesi F, Scherpereel A, Rittmeyer A, et al. Randomized phase III trial of maintenance bevacizumab with or without pemetrexed after first-line induction with bevacizumab, cisplatin, and pemetrexed in advanced nonsquamous non-small-cell lung cancer: AVAPERL (MO22089). J Clin Oncol. 2013;31(5):3004-3011.
- Barlesi F, Scherpereel A, Gorbunova V, et al. Maintenance bevacizumab-pemetrexed after first-line cisplatin-pemetrexed-bevacizumab for advanced nonsquamous nonsmall-cell lung cancer: updated survival analysis of the AVAPERL (MO22089) randomized phase III trial. Ann Oncol. 2014;25(5):1044-1052.
- Westeel V, Quoix E, Moro-Sibilot D, et al. Randomized study of maintenance vinorelbine in responders with advanced non-small-cell lung cancer. J Natl Cancer Inst. 2005;97(7):499-506.
- Fidias PM, Dakhil SR, Lyss AP, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(4):591-598.
- Ciuleanu T, Brodowicz T, Zielinski C, et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomized, double-blind, phase 3 study. Lancet. 2009;374(9699):1432-1440.