With increasing availability of CT scans and strong evidence for the utility of low-dose CT screening in high-risk populations,1
an increasing number of small lung nodules are being incidentally discovered. Many are small, subsolid, and may harbor premalignant or early-stage tumors. Local treatment of early primary lung cancers and lung oligometastases is gaining popularity, especially in patients with surgical contraindications.2
Local therapies include stereotactic body radiation therapy and multiple forms of thermal energy, including radiofrequency, microwave, and cryotherapy. Traditionally, thermal ablation was performed percutaneously under CT guidance but this can carry an 11% to 52% risk of pneumothorax3
and bronchopleural fistula,4
in addition to a 0.3% to 0.7% risk of tumor seeding along the catheter tract.5
,6
As an energy source, microwave energy is superior to radiofrequency because it is less affected by the high impedance of lung tissue, and thus able to produce a larger and more predictable ablation zone.7
Combining the best of two worlds, transbronchial microwave ablation (TBMA) has been recently developed, to take advantage of microwave energy’s ability to create a better ablation profile and to avoid pleural puncture. Another benefit of TBMA is its ability to reach certain regions of the lung that are otherwise difficult or dangerous for a percutaneous route, for instance, areas near the mediastinal pleura or diaphragm or at the lung apex and areas shielded by the scapula. Our institute performs TBMA using electromagnetic navigation bronchoscopy (ENB) for navigation and a localization tool in the hybrid operating room, with cone-beam CT (CBCT) and fluoroscopy support. After the locatable guide of ENB is navigated to the proximity of the target lung nodule, a needle is deployed. The locatable guide is exchanged with the ablation catheter, which is unsheathed by retracting the extended working channel. Transbronchial access tools may be required if the bronchus sign is not present. With CBCT, the predicted ablation zone can be accurately determined, to ensure adequate coverage of the lung nodule.
To date, we have performed TBMA on 65 nodules in 56 patients,8
with a mean maximal diameter of 15.3 mm, ranging from 7 to 29 mm. The technical success rate was 100%, although 18.4% of cases required planned or unplanned double ablation to ensure adequate margin, which had a mean of 5.4 mm. Length of hospital stay was short, with 76% discharged on 1 day after ablation and 95% discharged within 3 days. Complication rates were low, including mild pain not requiring hospitalization (17%), pneumothorax with or without drainage (7.7%), fever and/or post-ablation reaction (4.9%), self-limiting hemoptysis (3.1%), and bronchopleural fistula (1.5%). At a median follow-up of 14 months, there were two cases of local recurrence accompanied by systemic recurrence. Overall, TBMA has proven to be a safe, feasible, and promising local ablation technique with a local control rate comparable to other modalities, and lower pleural-based complications than those associated with percutaneous ablation.
Nevertheless, TBMA is not without its limitations. Numerous studies have correlated a risk of local recurrence with nodule size.9
,10
,11
The typical early post-ablative morphology of concentric ground glass opacities, in fact, contains an outer rim of congested lung tissue that retains viability12
; hence, the size seen on CT overestimates the area of true coagulation necrosis by 4.1 mm.13
Therefore, for example, an energy setting that has predicted ablation zone diameter of 2.5 cm, the maximal lesion diameter for a single ablation to achieve a margin of at least 5 mm is 1.5 cm. In fact, because of difficulty in positioning the ablation catheter in the center of the lesion, double ablation is likely required for lesions larger than 2 cm, especially when the bronchus sign is not present. Case selection for TBMA must also take nodule location into account. Care should be taken not to ablate nodules at the most apical aspect of the lung or abutting the mediastinum, in order to avoid brachial plexus, phrenic nerve, or vascular thermal injury. Whereas 66.7% of the lung nodules in our cohort were within 1 cm of the pleura or fissures, the majority of cases with ablated pleura did not develop pneumothorax.8
Therefore, by paying extra attention to avoid inadvertent pleural puncture, pneumothorax risk can be minimized. We have also noted that the actual ablation zone volumes were smaller by a mean of 21.4% compared with those predicted, which was likely due to variations in lung properties between human lungs and porcine lung models or to differences in impedance between normal and tumorous lung. Fortunately, this can be partially offset by the fact that tissue contraction occurs during ablation.14
Clinicians should take the contraction phenomenon into account when determining ablation margins. Close CT surveillance every 3 to 6 months to rule out local recurrence is crucial, as recurring lesions can be re-ablated.15
Although PET-CT may provide the best clue of early recurrence, it is expensive and not widely available. CT with contrast supplemented by nodule densitometry is a reasonable alternative.16
Recently, TBMA has been performed with mobile C-arm machines that can produce 3D reconstructions, so that a hybrid operating room setting with floor-mounted CBCT is no longer a prerequisite. In our experience, this should be reserved for larger and more solid lesions, as the CT resolution is lower. Several robotic bronchoscopic platforms have gained U.S. Food and Drug Administration approval in recent years, paving the way to easier, more intuitive, and semi-automated TBMA approaches. Ablation using other forms of energy—for example, thermal vapor—for lung cancer also has high potential; it is well-tolerated by patients and is able to create large, uniform ablation zones.17
The future of TBMA is promising and exciting: together with ENB navigation and biopsy, TBMA in the hybrid operating room is capable of providing one-stop diagnosis and treatment of malignant lung nodules.18
Figure. Pre- and post-ablation CBCT images of treated lung lesion
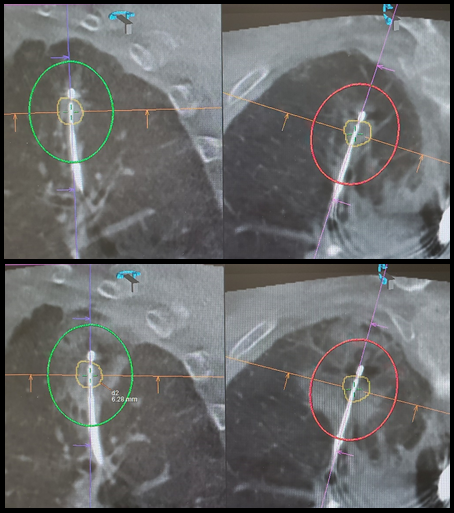
The upper panel shows the pre-ablation CBCT in two axes. Target lung nodule is marked by yellow tracings; the predicted ablation zone is represented by the red and green ovals. The lower panel shows the post-ablation CBCT, with ground-glass changes representing the actual ablation zone, which achieved a minimal margin of 6.3 mm.
- 1. National Lung Screening Trial Research Team; Aberle DR, Adams AM, Berg CD, et al. Reduced Lung-Cancer Mortality with Low-Dose Computed Tomographic Screening. N Engl J Med. 2011;365(5):395-409.
- 2. Zhao ZR, Lau RWH, Ng CSH. Catheter-based alternative treatment for early-stage lung cancer with a high-risk for morbidity. J Thor Dis. 2018;10:S1864-S1870.
- 3. Hiraki T, Gobara H, Fujiwara H, et al. Lung cancer ablation: Complications. Semin Intervent Radiol. 2013;30(2):169-175.
- 4. Zheng A, Yang X, Ye X, et al. Bronchopleural fistula after lung ablation: Experience in two cases and literature review. Indian J Cancer. 2015;52(6):e41-46.
- 5. Hiraki T, Mimura H, Gobara H, et al. Two Cases of Needle-Tract Seeding after Percutaneous Radiofrequency Ablation for Lung Cancer. J Vasc Interv Radiol. 2009;20(3):415-418.
- 6. Yamakado K, Akeboshi M, Nakatsuka A, et al. Tumor seeding following lung radiofrequency ablation: A case report. Cardiovasc Intervent Radiol. 2005;28(4):530-532.
- 7. Brace CL, Hinshaw JL, Laeseke PF, Sampson LA, Lee FT. Pulmonary thermal ablation: Comparison of radiofrequency and microwave devices by using gross pathologic and CT findings in a swine model. Radiology. 2009;251(3):705-711.
- 8. a. b. Chan JWY, Lau RWH, Ngai JCL, et al. Transbronchial microwave ablation of lung nodules with electromagnetic navigation bronchoscopy guidance—a novel technique and initial experience with 30 cases. Transl Lung Cancer Res. 2021;10(4):1608-1622.
- 9. Dupuy DE, Fernando HC, Hillman S, et al. Radiofrequency ablation of stage IA non-small cell lung cancer in medically inoperable patients: Results from the American College of Surgeons Oncology Group Z4033 (Alliance) trial. Cancer. 2015;121(19):3491-3498.
- 10. Healey TT, March BT, Baird G, Dupuy DE. Microwave Ablation for Lung Neoplasms: A Retrospective Analysis of Long-Term Results. J Vasc Interv Radiol. 2017;28(2):206-211.
- 11. Vogl TJ, Worst TS, Naguib NNN, Ackermann H, Gruber-Rouh T, Nour-Eldin NEA. Factors influencing local tumor control in patients with neoplastic pulmonary nodules treated with microwave ablation: A risk-factor analysis. Am J Roentgenol. 2013;200(3):665-672.
- 12. Chheang S, Abtin F, Guteirrez A, Genshaft S, Suh R. Imaging features following thermal ablation of lung malignancies. Semin Intervent Radiol. 2013;30(2):157-168.
- 13. Yamamoto A, Nakamura K, Matsuoka T, et al. Radiofrequency Ablation in a Porcine Lung Model: Correlation Between CT and Histopathologic Findings. Am J Roentgenol. 2005;185(5):1299-1306.
- 14. Kim C. Understanding the nuances of microwave ablation for more accurate post-treatment assessment. Futur Oncol. 2018;14(17):1755-1764.
- 15. Yang X, Ye X, Huang G, et al. Repeated percutaneous microwave ablation for local recurrence of inoperable Stage I nonsmall cell lung cancer. J Cancer Res Ther. 2017;13(4):683-688.
- 16. Abtin FG, Eradat J, Gutierrez AJ, Lee C, Fishbein MC, Suh RD. Radiofrequency ablation of lung tumors: Imaging features of the postablation zone. Radiographics. 2012;32(4):947-969.
- 17. Scott Ferguson J, Henne E. Bronchoscopically Delivered Thermal Vapor Ablation of Human Lung Lesions. J Bronchol Interv Pulmonol. 2019;26(2):108-113.
- 18. Chan JWY, Yu PSY, Lau RWH, Ng CSH. Hybrid operating room—one stop for diagnosis, staging and treatment of early stage NSCLC. J Thor Dis. 2020;12(2):123-131.






