Myung-Ju Ahn, MD, PhD
The discovery of EGFR mutation and the subsequent development of EGFR TKIs provided a prototype of personalized medicine in the treatment of NSCLC. Until now, many first-, second-, and third-generation EGFR TKIs used as monotherapy or in combination with an antiangiogenic agent or chemotherapy significantly improved PFS.
In the FLAURA study, PFS was 18.9 months with osimertinib compared to 10.2 months with standard of care in the first line. However, most patients experienced progression over time because of acquired resistance.1
Various mechanisms of resistance to EGFR TKIs have been identified, including on-target and off-target. Although there are many clinical trials underway regarding these resistance mechanisms, only one third-generation EGFR TKI has been approved by the U.S. FDA for the treatment of patients who develop T790M mutation after treatment with first- or second-generation EGFR TKIs.2
Whereas immune checkpoint inhibitors (ICIs) have changed the treatment paradigm in NSCLC, the role of ICIs is limited in EGFR-mutant NSCLC, largely because of low tumor mutational burden and a non-inflamed tumor microenvironment. In contrast, the role of VEGF and antiangiogenic agents in combination with immunotherapy is being revisited. VEGF signaling plays a central role in tumor vasculature development and maintenance, and it can also lead to a suppressive tumor microenvironment.
Previously, a subgroup analysis of IMpower150 demonstrated that PFS and OS were improved with combination atezolizumab/bevacizumab/carboplatin/paclitaxel compared to bevacizumab/carboplatin/paclitaxel in patients with EGFR-mutant NSCLC (PFS HR 0.61; OS HR 0.61).3 A recently updated subgroup analysis with long-term follow-up demonstrated that OS was significantly improved with a four-drug regimen in patients with sensitizing EGFR mutation (HR 0.6)4. However, given that the number of patients with EGFR mutation represented only 9% of the total study population, a large-scale randomized study is needed to confirm the role of ICI plus antiangiogenic agents in patients with actionable EGFR mutations.
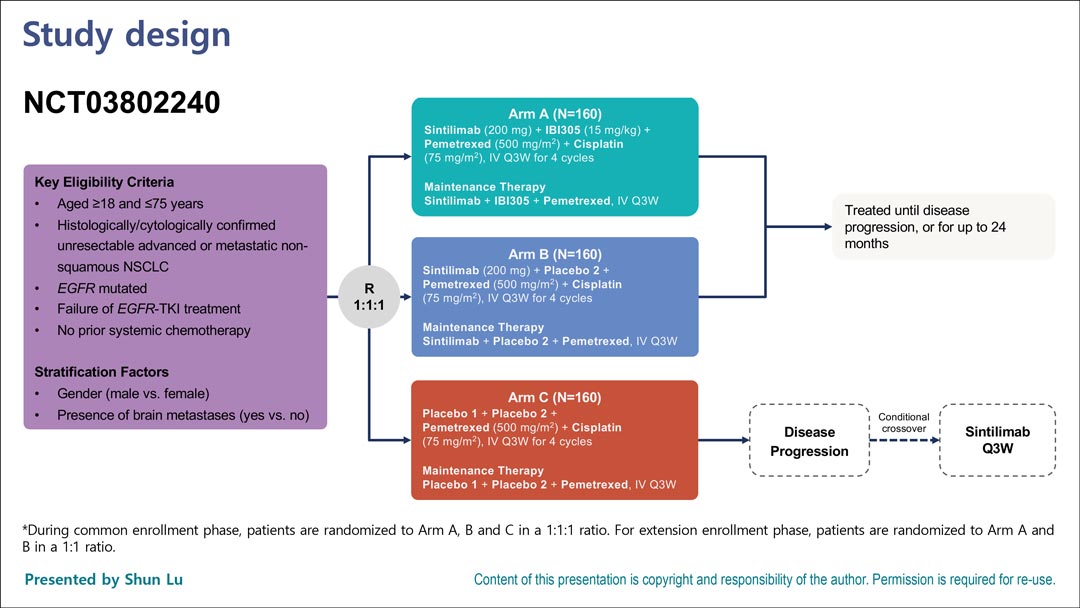
In a virtual plenary session at the ESMO Congress 2021, Shun Lu, MD, PhD, presented an interim analysis of the randomized phase III ORIENT-31 study5, which has three arms (Fig. 1). Patients with EGFR mutation and patients with EGFR mutation whose disease did not respond to TKIs (including osimertinib) were randomly assigned to either sintilimab (an anti–PD-1 antibody developed in China) plus IBI305 (a bevacizumab biosimilar) plus pemetrexed/cisplatin, to sintilimab plus pemetrexed/cisplatin, or to pemetrexed/cisplatin alone. The primary endpoint was PFS by blinded independent review committee.
Interim analysis demonstrated that the four-drug regimen, sintilimab/IBI305/pemetrexed/cisplatin, significantly improved PFS compared to pemetrexed/cisplatin/placebo. Median PFS was 6.9 months for the four-drug regimen versus 4.3 months for pemetrexed/cisplatin (Fig. 2; HR 0.464). Two Kaplan-Meier curves of PFS separated very early.
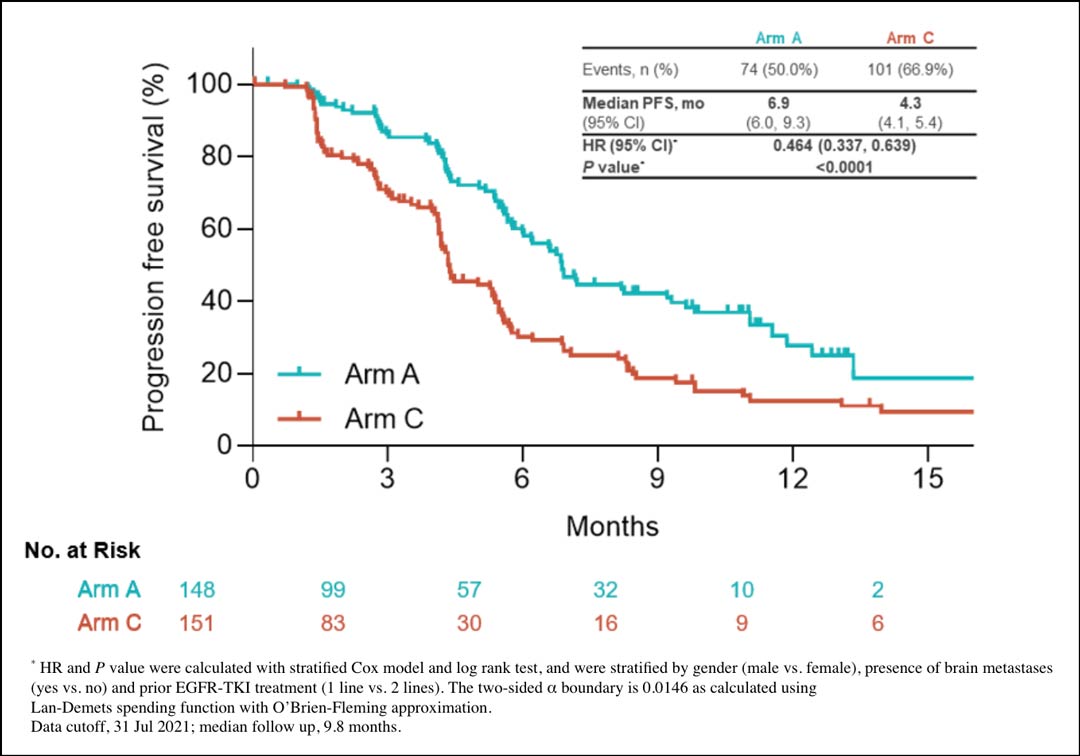
Fig. 2. Combination Sintilimab/IBI305/Pemetrexed/Cisplatin Improves PFS Compared to Combination Pemetrexed/Cisplatin/Placebo by Interim Analysis. Figure courtesy of Dr. Shun Lu.
Accordingly, the overall response rate (ORR) was 43.9% for the four-drug regimen versus 25.2% for pemetrexed/cisplatin. However, the duration of response was 8.3 months versus 7.0 months—not such a significant difference. PFS improvement was observed across most subgroups, but less benefit was observed in the second-line EGFR-TKI treatment group. Compared to pemetrexed/cisplatin as a control, the four-drug regimen was associated with increased grade 3 treatment-related adverse events, a greater discontinuation rate, and increased, but manageable immune-related adverse events.
Although this is only an interim analysis, it is the first large randomized study to demonstrate improvement of PFS with chemotherapy/anti–PD-1/antiangiogenic agent compared to pemetrexed/cisplatin in patients with NSCLC whose disease did not respond to EGFR TKI treatment. Although there are limitations to direct comparison of ORIENT-31 with IMpower150, there are several important differences:
- Pemetrexed/cisplatin was administered as the chemotherapy agent in ORIENT-31, versus carboplatin/paclitaxel in IMpower150.
- Anti–PD-1 agents were the form of ICI used for ORIENT-31, as opposed to anti–PD-L1 employed in IMpower150.
- The population of ORIENT-31 was younger and included more people who had never smoked and a greater number with EGFR
- ORIENT-31 allowed patients with T790M mutation who had been treated with third-generation EGFR TKIs, which accounted for 30% of patients, whereas IMpower150 enrolled only patients pretreated with first-line EGFR TKIs and included no data on T790M.
- Thirty-five percent of ORIENT-31 patients had brain metastases, whereas IMpower150 contained no data on brain metastases.
A numerically lower ORR and PFS in the four-drug regimen in ORIENT-31 compared to those of IMpower150 might be attributed to the approximately 30% of patients who received both first- and second-generation as well as third-generation TKIs—suggesting more heavily treated patients were enrolled.
The duration of response (8.3 months vs. 7.0 months) does not seem to be significantly better for the four-drug regimen despite a higher ORR (43.9% vs. 25.2%); this warrants long-term follow-up. The PFS benefit was observed regardless of brain metastases, which is promising. Further analysis such as intracranial ORR or intracranial PFS should be evaluated. Given that 30% of patients received a third-generation TKI, most of whom had T790M-positive disease, and that HR for PFS was 0.719 (0.432-1.194, greater than 1), the role of the four-drug regimen in these patient groups should be carefully interpreted and will require further follow-up.
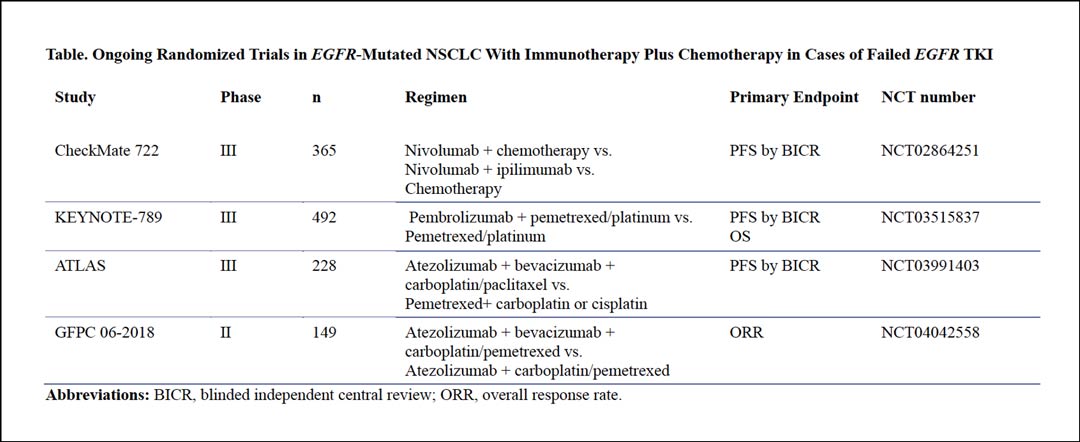
We look forward to seeing further analysis of the A versus B arms—whether the four-drug regimen is better than the three-drug regimen without antiangiogenic drug; additional analysis of the B versus C arms—whether immunotherapy plus chemotherapy is better than chemotherapy alone—is also needed. A final OS analysis showing whether PFS benefit can translate into long term survival benefit is needed, as is development of a biomarker indicating which patients are most likely to benefit from the four-drug regimen. Several other randomized phase III trials are ongoing in patients with EGFR-mutated NSCLC whose disease has not responded to EGFR TKIs (Table). The results of these studies are eagerly awaited.
In summary, ORIENT-31 is the first large, randomized phase III study to demonstrate improvement of PFS with combination sintilimab/IBI305/pemetrexed/cisplatin compared to pemetrexed/cisplatin, which confirms the role of antiangiogenic agents with ICI combined with chemotherapy in EGFR-TKI–refractory NSCLC. This four-drug regimen may be a reasonable option in patients with EGFR-mutated NSCLC whose disease has progressed on EGFR TKIs, including third-generation EGFR TKIs.
References
- 1. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med 2018; 378(2):113-125. Doi:10.1056/NEJMoa173137
- 2. U.S. FDA grants regular approval to osimertinib for the treatment of patients with metastatic EGFR T790 mutation-positive NSCLC
- 3. Reck M, Mok TSK, Nishio M, et al. Atezolizumab Plus Bevacizumab and Chemotherapy in Non-Small Cell Lung Cancer (IMpower150): Key Subgroup Analyses of Patients with EGFR Mutations or Baseline Liver Metastases in a Randomized, Open-Label Phase 3 Trial. Lancet Respir Med 2019;7(5):387-401. doi:10.1016/S2213-2600(19)30084-0
- 4. Nogami N, Barlesi F, Socinski MA, et al. IMpower150 Final Exploratory Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in Key NSCLC Patient Subgroups with EGFR Mutation or Metastases in the Liver or Brain. J Thorac Oncol 2022;17(2):309-323. doi: 10.1016/j.jtho.2021.09.014
- 5. Lu S, Wu L, Jian H, et al: VP9-2021: ORIENT-31: Phase III study of sintilimab with or without IBI305 plus chemotherapy in patients with EGFR mutated nonsquamous NSCLC who progressed after EGFR-TKI therapy. 2021 ESMO Virtual Plenary. Abstract VP9-2021. Presented November 19, 2021.