The primary results of the AEGEAN trial of perioperative durvalumab plus adjuvant chemotherapy versus chemotherapy alone in early-stage non-small cell lung cancer (NSCLC) showed statistically significant and clinically meaningful benefits in efficacy from combination treatment. Surgical results from the trial showed no significant differences in surgical approaches, outcomes, or safety profiles between the two arms.

“With clinically meaningful improvements in efficacy, no adverse impact on surgical outcomes, and a manageable safety profile, the addition of perioperative durvalumab to neoadjuvant chemotherapy is a potential new treatment option for patients with resectable NSCLC,” said Tetsuya Mitsudomi, MD, PhD, Professor of Thoracic Surgery, Kindai University Faculty of Medicine, Osaka-Sayama, Japan.
Dr. Mitsudomi discussed the surgical outcomes of neoadjuvant plus adjuvant durvalumab plus chemotherapy versus chemotherapy alone during an oral abstract session at the 2023 World Conference on Lung Cancer. The session, “Pushing the Boundaries: Adjuvant and Neoadjuvant Approaches in Early Stage Non-Small Cell Lung Cancer” took place on Monday, September 11, and is available on demand for registered WCLC 2023 attendees through December 31.
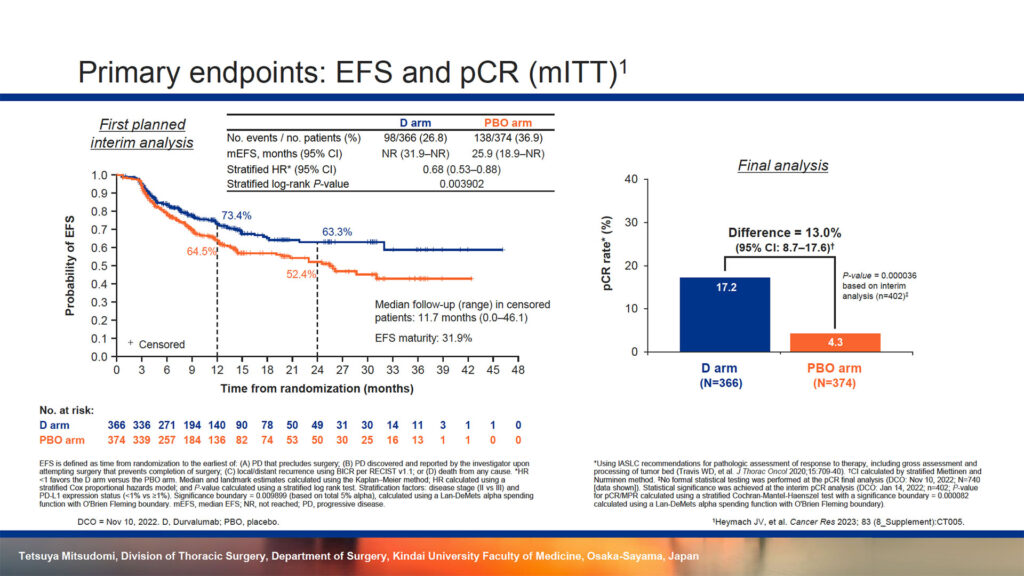
Surgery remains the cornerstone of treatment for early-stage NSCLC, Dr. Mitsudomi said. However, a growing body of evidence suggests that many patients may benefit from neoadjuvant and adjuvant pharmacotherapy. There is good evidence that perioperative chemotherapy can improve overall outcomes in resectable NSCLC and durvalumab has become standard of care for patients with unresectable NSCLC. It was not clear whether patients with resectable NSCLC might benefit from combination durvalumab plus chemotherapy. AEGEAN randomized 802 patients at 222 centers in 28 countries to neoadjuvant durvalumab plus chemotherapy and adjuvant durvalumab or neoadjuvant placebo plus chemotherapy and adjuvant placebo. Efficacy analyses were performed on a modified intent-to-treat population that included 740 patients. The primary results showed a hazard ratio of 0.68 for event free survival (p=0.003902) and a 13% advantage in pathologic complete response (p=0.000036) for the combination arm.
In the combination arm, 74.8% of patients successfully completed neoadjuvant treatment compared to 87.2% of patients receiving chemotherapy alone. About 20% of patients in each arm did not undergo surgery. More patients with stage II disease underwent and completed surgery compared to those with stage III disease.
Patients who completed neoadjuvant treatment a similar median time from the last neoadjuvant treatment to surgery (34 days) and from surgery to the first adjuvant treatment (51 days) across the two arms. There were similar delays in surgery between the arms and similar reasons for delay, including logistical difficulties, adverse events, unresolved toxicities from earlier treatment on study, among others.
Surgical approaches were similar between the two arms, about 90% open and 10% minimally invasive. Most patients, about 85%, underwent lobectomy, about 9% pneumonectomy, and the balance another procedure. About 85% of patients in both arms had mediastinal lymph node dissection.
Resection status was also similar, over 90% R0 regardless of stage, although the combination arm showed numerically superior R0 results. R1 and R2 resections were similar across both arms.
About 40% of patients in both arms reported any adverse event related to surgery with 8.5% grade 3/4 and 11% serious adverse events. The most common adverse events included procedural pain, wound complications, pneumonia, anemia, and pneumothorax.
“Regardless of disease stage, the addition of perioperative durvalumab did not adversely impact the feasibility, type, approach, or timing of surgery in patients with resectable NSCLC,” Dr. Mitsudomi said. “Perioperative durvalumab resulted in numerically higher R0 resection rates.”