Biomarkers that can help predict how a patient will respond to specific therapies are an invaluable clinical tool for improving patient selection and therapeutic outcomes. Lung cancer has long been the prototype for the discovery of novel biomarkers and marker-guided targeted treatments.
In the first of two Presidential Symposia during the 2024 World Conference on Lung Cancer, Marina Chiara Garassino, MD, Professor of Medicine at the University of Chicago, shared exciting data on the development of a pioneering tool for quantifying and locating trophoblast cell surface antigen 2 (TROP2) in lung tumor specimens.
In prior analyses of TROPION-Lung01, a pivotal phase III study, the TROP2-targeted antibody-drug conjugate (ADC) datopotamab deruxtecan (dato-DXd) significantly improved progression-free survival (PFS) compared to docetaxel, the standard of care, for patients with locally advanced or metastatic non-small cell lung cancer (NSCLC) treated in the second line setting.
Dato-DXd is comprised of an anti-TROP2 antibody tethered to a topoisomerase 1 inhibitor with cytotoxic activity via a plasma-stable linker. Unlike other anti-TROP2 ADCs, dato-DXd must bind to membrane-bound TROP2 and be internalized before the payload can be delivered and cancer cells are killed. TROP2 is widely expressed in lung cancer cells, both on the cell surface and in the cytoplasm.

While other anti-TROP2 ADCs have previously demonstrated efficacy in treating patients with NSCLC, Dr. Garassino explained the rationale for developing a new pathology strategy.
“Conventional immunohistochemistry scoring has not predicted response to TROP2-directed ADCs in patients with NSCLC,” she said. “For this reason, we hypothesized that a more precise and quantitative assessment of TROP2 expression on the cell membrane and cytoplasm could predict efficacy of dato-DXd in patients with NSCLC.”
To differentiate membrane-bound and cytoplasmic TROP2 in NSCLC tissue specimens, Dr. Garassino and colleagues developed a fully supervised computational pathology tool—Quantitative Continuous Scoring-Normalized Membrane Ratio or QCS-NMR. The tool uses AI-enabled automated image analysis to quantitatively score how much TROP2 is membrane-bound, relative to the total TROP2. The TROP2 QCS-NMR score provides a new way to stratify patients; the lower the score, the higher the cytoplasmic proportion of TROP2.
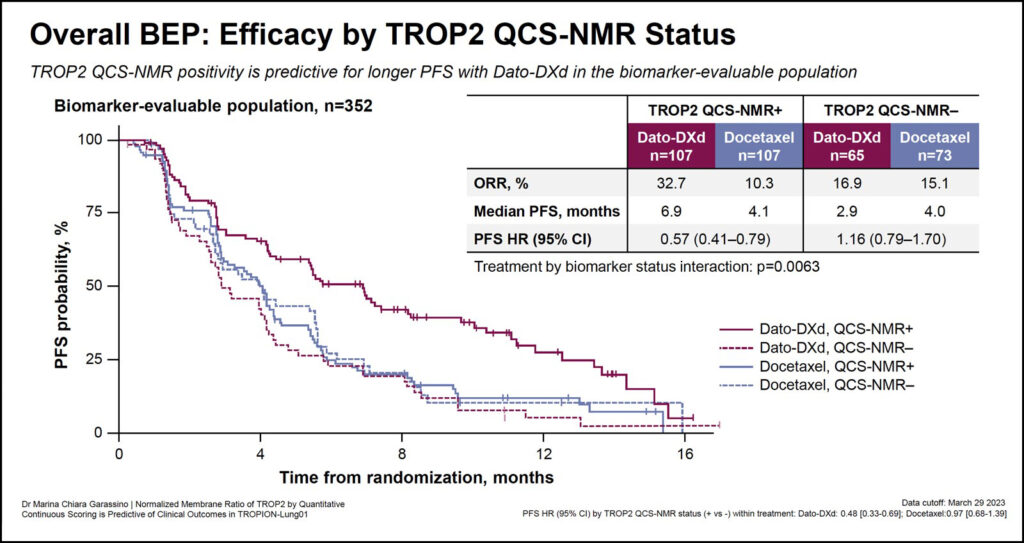
Dr. Garassino and colleagues used TROP2 NMR scores to stratify patients into two groups—TROP2-positive, with ≥75% of tumor cells with TROP NMR ≤0.56 and TROP2-negative, with <75% of tumor cells with TROP2 NMR ≤0.56.
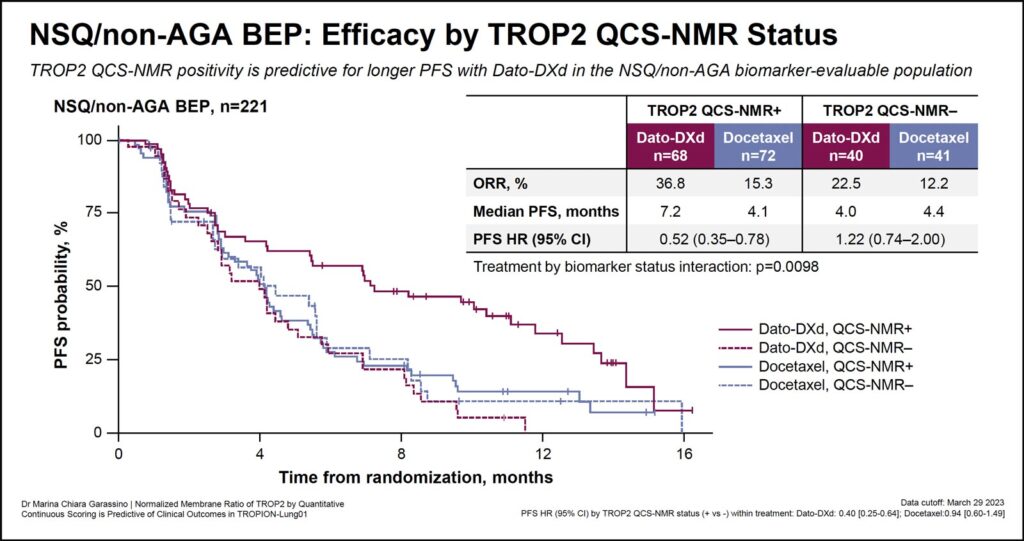
When this TROP2 scoring method was applied to samples from patients in the TROPION-Lung01 study with biomarker-evaluable tissue samples, TROP2-positive prevalence was higher in patients with nonsquamous histology (66%), compared to those with squamous histology (44%).
Delivering the results, Dr. Garassino said, “TROP2 QCS-NMR positivity is predictive of longer PFS—median PFS is 6.9 months in TROP2-positive patients compared to 2.9 months in TROP2-negative patients—in the biomarker-evaluable population.”
Moreover, the overall response rate was higher in the TROP2-positive cohort (32.7%) compared to the TROP2−negative (16.9%) patients.
Dr. Garassino concluded, “TROP2 QCS-NMR has the potential to be the first TROP2 biomarker and the first computational pathology biomarker to predict clinical response to dato-DXd in NSCLC.”
Discussant Sanja Dacic, MD, PhD, Professor of Pathology and Vice Chair and Director of Anatomic Pathology at Yale School of Medicine, New Haven, Connecticut, said any new assay development requires comparison to the gold standard.
Unfortunately for ADCs, in general, and TROP2, in particular, previous attempts to develop new assays have failed, she said.
“What is really exciting about this assay is that we are essentially using the supervised computational pathology approach to quantify TROP2,” Dr. Dacic said. “There is no gold standard to which we can compare this assay.”
She added that this new tool is “shaking up” the current pathology workflow and approach to biomarker evaluation in lung cancer specimens.