As reported in November 2023, the FLAURA-2 trial demonstrated an improvement in progression-free survival (PFS) with first-line osimertinib plus chemotherapy in patients with EGFR-mutated advanced non-small cell lung cancer (NSCLC) compared to osimertinib monotherapy.1 Now, new analyses from FLAURA-2 show additional benefits for the combination therapy.

During the recent 2024 European Lung Cancer Congress, organized by the IASLC and the European Society for Medical Oncology, Natalia Valdiviezo, MD, Department of Oncology, National Institute of Neoplastic Diseases, Surquillo, Peru, presented post-progression outcomes data as well as data from a second interim overall survival (OS) analysis. The meeting took place March 20-23 in Prague, Czech Republic.
Dr. Valdiviezo provided an overview of the post-progression outcomes, which she said help to further contextualize the potential long-term efficacy of the osimertinib plus chemotherapy combination beyond PFS. These secondary endpoints include time to first subsequent therapy (TFST) or second-line PFS, time to second subsequent therapy (TSST), and overall survival.
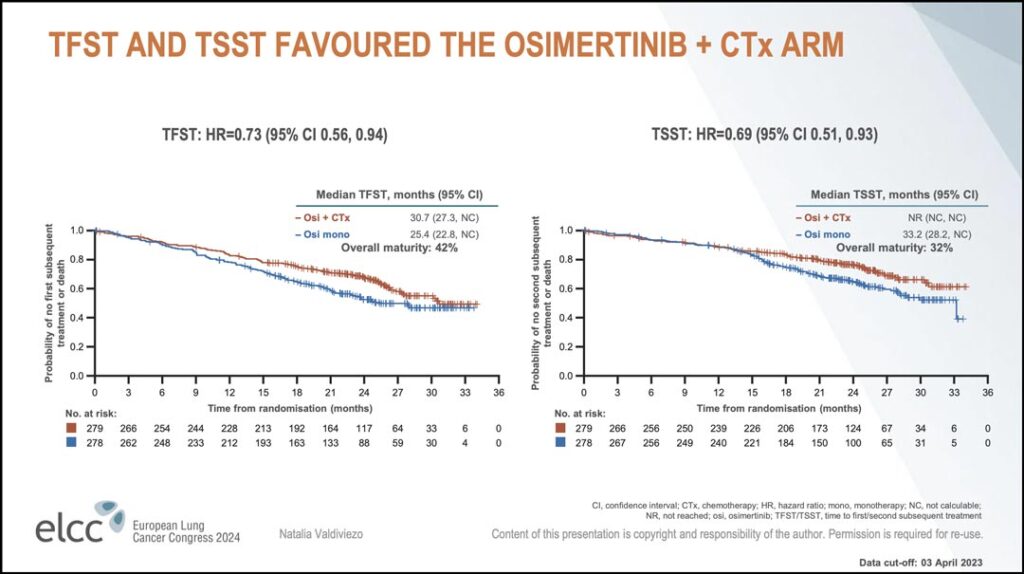
“Approximately 85% of the patients in each arm continued osimertinib treatment after progression,” Dr. Valdiviezo said. “Among them, approximately 50% of the patients in each arm remained on osimertinib for 2 months or longer. As you can see in the Kaplan-Meier curves, the hazard ratio for both time to first subsequent therapy and time to second subsequent therapy favor the osimertinib plus chemotherapy arm.” (see Figure 1)
Additionally, she said with a hazard ratio of 0.70, the results of the second progression free survival analysis indicate that those on osimertinib plus chemotherapy reduced the risk of second progression or death by 30% compared with those on osimertinib monotherapy.
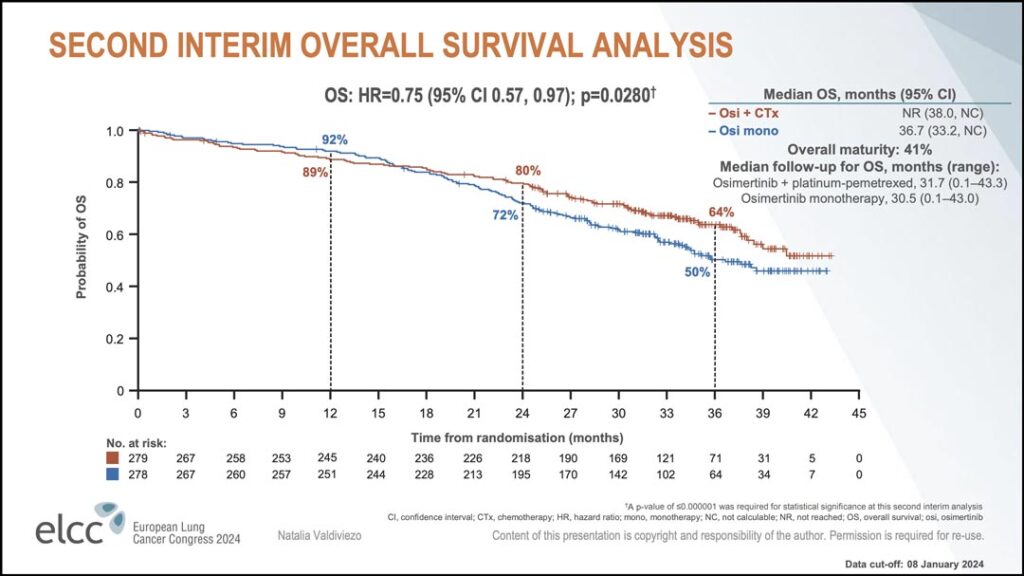
Since the primary data cutoff, a second interim analysis for OS has also been conducted. With overall maturity of 41% the hazard ratio for OS was 0.75.
“Looking at the Kaplan-Meier plot, even though osimertinib monotherapy showed good efficacy, we can see the separation of the curves in favor of the combination treatment,” Dr. Valdiviezo said. (see Figure 2) “These data support a favorable trend in OS with osimertinib plus chemotherapy. The final analysis for OS will be conducted at approximately 60% maturity.”
She also shared results from an exploratory analysis of the second interim OS data according to the predefined subgroups for the primary PFS analysis. Overall survival benefit with osimertinib plus chemotherapy versus osimertinib monotherapy was consistent across the subgroups and most of the hazard ratio estimates were below 0.8.
“The emerging data again demonstrate encouraging results with increased benefit realized over time,” Dr. Valdiviezo said.
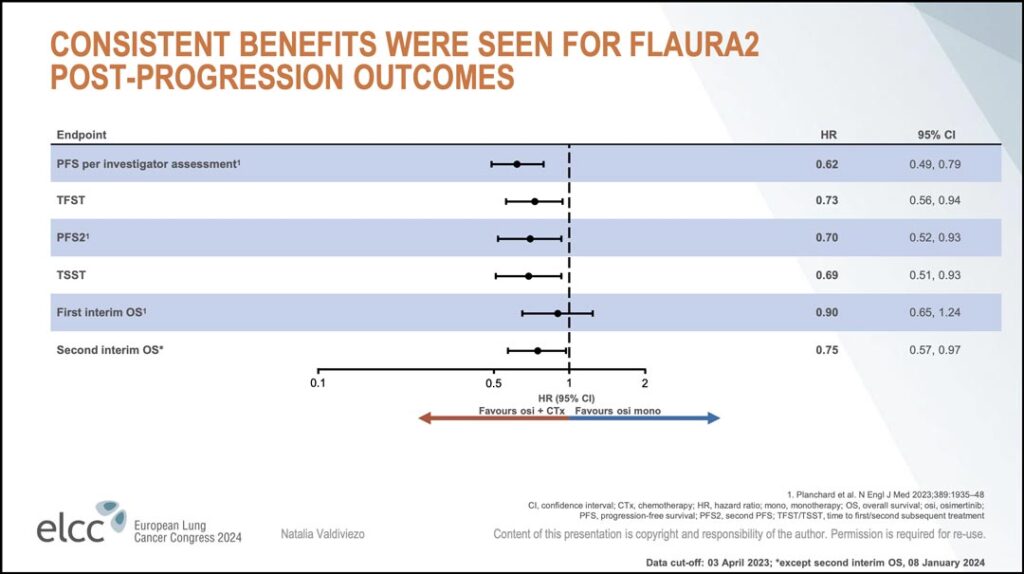
Finally, she shared a summary of the hazard ratios for FLAURA-2 post-progression outcomes together with their 95% confidence intervals. (see Figure 3)
“These hazard ratios provide a more robust characterization of the data,” she said. “The hazard ratio estimates and upper confidence intervals for TFST, PFS2, and TSST all demonstrate consistency between the post-progression outcomes and OS endpoints. There is a shift in favor of the osimertinib plus chemotherapy arm…”
“We can conclude that first-line osimertinib plus platinum-pemetrexed chemotherapy provides long-term, clinical meaningful benefit beyond that seen from the initial PFS for patients with EGFR-mutant advanced non-small cell lung cancer.”
References
- 1. Planchard D, Jänne PA, Cheng Y, et al. Osimertinib with or without Chemotherapy in EGFR-Mutated Advanced NSCLC. N Engl J Med. 2023;389(21):1935-1948. doi:10.1056/NEJMoa2306434