Although low-dose CT scans enable the detection of very small lung nodules in individuals who face a high risk of developing lung cancer, it is often impossible to quickly determine whether the lesions are malignant due to their size or location.
Technology designed to enhance the depth of information available when reading a CT scan could reduce the length of follow up in patients with early NSCLC, allowing them to begin treatment sooner, while helping to prevent the performance of unnecessary invasive tests.
Being investigated for those uses are a number of assays that incorporate factors such as clinical characteristics, patterns of DNA methylation or fragmentation, exosome antigens, circulating tumor cells, circulating free DNA, and radiomic features. One such study conducted in China and presented during the IASLC’s 2021 World Conference on Lung Cancer (Abstract OA16.04) tested PulmoSeek Plus, a combination of clinical, imaging, and blood-based DNA methylation biomarkers that shows promise in improving the diagnostic power of low-dose CT.
So far, the only blood-based predictive model approved by the U.S. Food and Drug Administration is Galleri, which analyzes methylation patterns of cell-free DNA in the blood to diagnose 50 cancer types. But when disease is in its earliest stages, the sensitivity of the test drops significantly, noted Christian Rolfo, MD, PhD, director of clinical research at the Center for Thoracic Oncology at Mount Sinai’s Tisch Cancer Institute in New York.
The assay findings presented during the conference by Dr. Liang et al, on the other hand, demonstrated a sensitivity of more than 95% for stage I lung cancers, Dr. Rolfo noted.
“They integrated something that we need to integrate — the liquid biopsy and a radiological model — to improve the sensitivity of the low-dose CT scan, which is weak in finding all the patients who need treatment,” he said. “In addition, there are patients around the world who do not have access to CT screening, so having another tool that is able to identify early lung cancer is a good idea.”
Study Details
The study was presented by senior author Jianxing He, MD, of the 1st Affiliated Hospital of Guangzhou Medical University in China.
Having previously created and validated a blood-based DNA methylation model known as PulmoSeek,1
,2
his team dubbed their novel combination test PulmoSeek Plus.
In conducting their prospective-specimen collection and retrospective-blinded-evaluation trial, they enrolled 1,097 patients with no previous cancer history, each of whom had a solitary, CT-detected pulmonary nodule 5 mm to 30 mm in diameter. After a training set of 839 patients was tested using a model incorporating clinical and imaging biomarkers (CIBM), 258 patients (214 of them having malignant nodules) were assessed using the CIBM and PulmoSeek models along with CT screening. Together, these techniques comprised PulmoSeek Plus.
Based on their receiver operating characteristic curves, the diagnostic performance of PulmoSeek Plus was compared against that of the CIBM, PulmoSeek, and the Mayo and Brock models, which, like the CIBM, predict risk based on clinical and CT characteristics. Pathologic diagnosis was used as the gold-standard comparator.
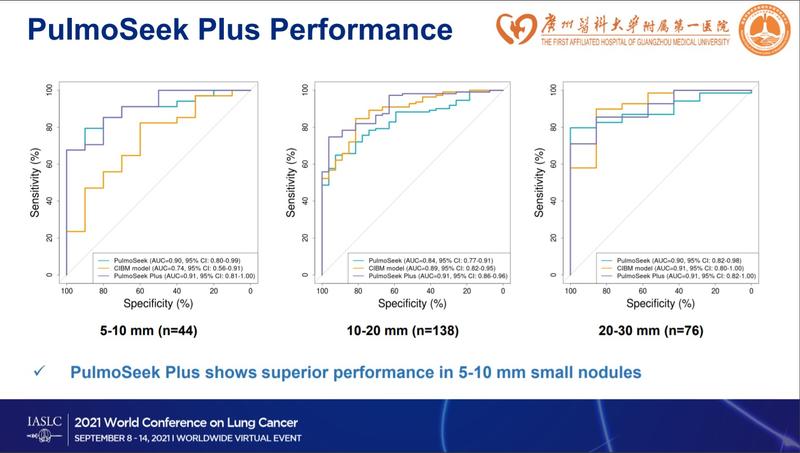
Areas under the curve for the CIBM, PulmoSeek, and PulmoSeek Plus models in the validation set were 0.847 (95% CI: 0.803-0.891), 0.869 (95% CI: 0.828-0.910), and 0.911 (95% CI: 0.876-0.946), respectively, Dr. He reported. All three models showed better accuracy than Mayo (AUC = 0.60, 95% CI: 0.52-0.68) and Brock (AUC=0.70; 95% CI: 0.63-0.77).
The sensitivity of the CIBM, PulmoSeek, and PulmoSeek Plus models was 91.6% (95% CI: 0.882-0.950), 90.7% (95% CI: 0.872-0.942), and 98.6% (95% CI: 0.972-1.000), respectively, he said. The sensitivity of PulmoSeek Plus in stage IA1 (n = 48), IA2 (n = 86), IA3 (n = 31), and IB (n = 24) was 97.9% (95% CI: 0.962-0.996), 98.8% (95% CI: 0.975-1.000), 96.8% (95% CI: 0.947-0.989), and 100.0% (95% CI: 1.00-1.00), respectively. PulmoSeek’s sensitivity was 100.0% (95% CI: 1.00-1.00) in subcentimeter nodules (n = 111), 98.2% (95% CI: 0.966-0.998) in 10-mm to 20-mm nodules (n = 69), and 98.6% (95% CI: 0.972-1.000) in 20-mm to 30-mm nodules (n = 34). Overall, PulmoSeek Plus showed significantly improved accuracy (90.3% [95% CI: 0.867-0.939]) compared with the CIBM and PulmoSeek models (84.5% [95% CI: 0.801-0.889] vs. 83.7% [95% CI: 0.792-0.882]).
When it came to predicting the invasiveness of lung adenocarcinoma, PulmoSeek was 85.8% accurate, Dr. He reported.
PulmoSeek Plus’s specificity was set at 50%.
“PulmoSeek Plus is an accurate tool for the early detection and classification of pulmonary nodules, potentially reducing the rate of unnecessary invasive procedures,” Dr. He concluded.
The Future of Liquid Biopsy
Discussant Lecia V. Sequist, MD, MPH, of Massachusetts General Hospital, called the PulmoSeek Plus results “impressive.”
Next, she said, a prospective study should test the assay’s clinical utility, specifically whether it can improve triage to biopsy and resection, cut down on unnecessary procedures, and increase the number of patients undergoing screening. The study should also explore the feasibility of introducing blood draws and scan annotation into lung screening, Dr. Sequist said.
Dr. Rolfo added that future studies should test the accuracy of the assay in different lung cancer histologies and validate the results in a larger population of patients that is ethnically diverse.
“In the future,” he said, “I think we will be able to find tools that are even better. Circulating-tumor DNA methylation could be an approach, but several groups are working beyond that by using a multiomics or metabolomics approach that would get the best of every technique and integrate them into screening.”
- 1. Liang W, Zhao Y, Huang W, et al. Non-invasive diagnosis of early-stage lung cancer using high-throughput targeted DNA methylation sequencing of circulating tumor DNA (ctDNA). Theranostics. 2019;9(7):2056-2070.
- 2. Liang W, Chen Z, Li C, et al. Accurate diagnosis of pulmonary nodules using a noninvasive DNA methylation test. J Clin Invest. 2021;131(10):e145973.