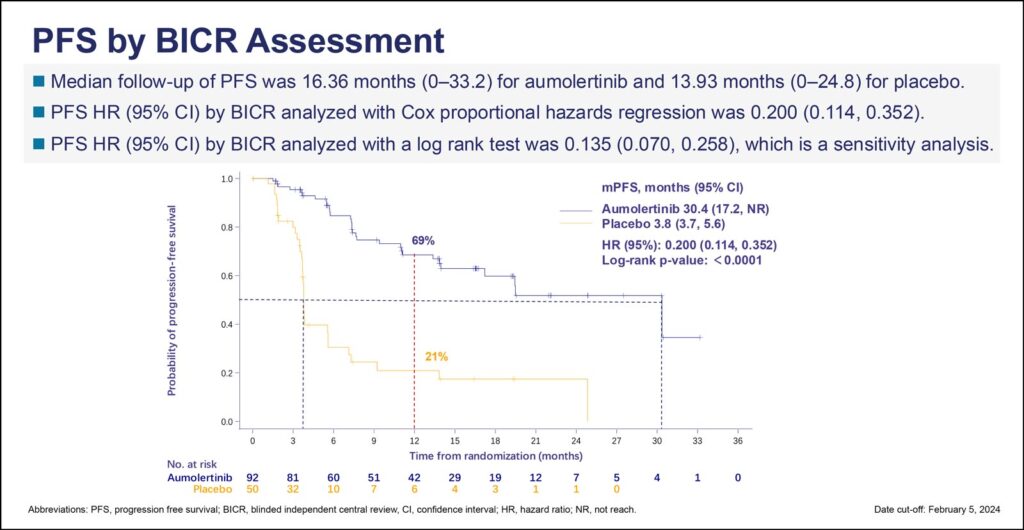
Interim results from the POLESTAR trial demonstrated the third-generation EGFR TKI aumolertinib improved progression-free survival (PFS) 80% compared to placebo for patients with unresectable stage III EGFR-mutated non-small cell lung cancer (EGFRm NSCLC) following chemoradiotherapy (CRT). The data, presented during the second of two Presidential Symposia during the 2024 World Conference on Lung Cancer, showed a median PFS of 30.4 months for patients taking the once-daily oral TKI versus 3.8 months for placebo (HR 0.200, 95% CI 0.114-0.352, p<0.0001).

“These findings suggest that aumolertinib may represent a novel treatment option as maintenance therapy for patients with unresectable stage III EGFR mutant NSCLC after CRT,” said Xiangjiao Meng, PhD, Director of the Fourth Ward of Chest Radiotherapy, Shandong Cancer Hospital and Institute, Shandong First Medical University, Taian, China. “The PFS benefit favoring aumolertinib was consistent across all predefined subgroups.”
Durvalumab is the standard of care for the majority of patients with locally advanced (LA) NSCLC without disease progression following CRT, Dr. Meng said, but there are very limited data on the potential benefit from consolidation immunotherapy for individuals with EGFRm NSCLC after CRT. Aumolertinib has shown efficacy in first- and second-line treatment of advanced EGFRm NSCLC and has been approved in China.
The POLESTAR trial randomized 147 patients—94 received aumolertinib and 53 were given a placebo. To be included in the trial, patients were required to have an actionable EGFR mutation (Ex19del or L858R), unresectable stage III NSCLC, and no evidence of progression after CRT. The median patient age was 59, about 50% were female, and 30% had no smoking history. Tumors were assessed every 8 weeks until week 48 and then every 12 weeks until progression.
Treatment continued until progression or discontinuation for toxicity or other reasons. Median follow-up was 16 months (range 0-33.2) for patients treated with aumolertinib and 14 months for those who received a placebo. The primary endpoint is PFS, and secondary endpoints include overall survival, overall response rates, duration of response, disease control rate, central nervous system PFS, and safety.
Overall survival data were immature at cutoff for the interim analysis. PFS as assessed by investigators was similar to the PFS assessed by the blinded independent review committee, although investigator assessment showed a slightly better hazard ratio of 0.150 (95% CI 0.080-0.284, p<0.0001).
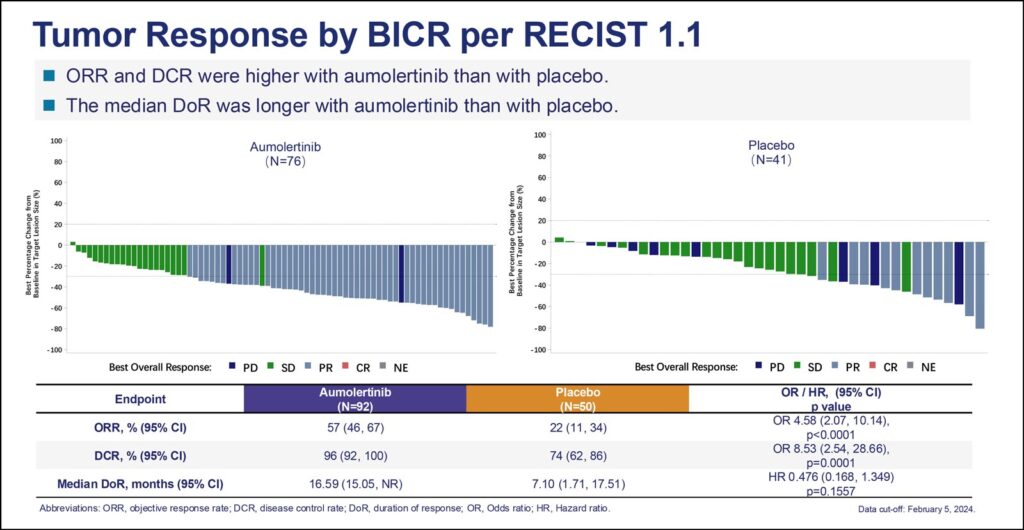
Overall response rate and disease control rates were higher for aumolertinib compared to placebo, 57% and 96% versus 22% and 74%, respectively. Median duration of response for aumolertinib was 17 months compared to 7 months for placebo.
In terms of efficacy, aumolertinib patients had significantly fewer sites of disease progression—21% versus 58.0%—including chest, brain, distant lymph node, abdomen, and bone lesions.
The safety profile for aumolertinib was tolerable and as expected based on earlier studies. The most common adverse event for aumolertinib was increased blood creatine phosphokinase, with most events grade 1 or 2. Aumolertinib was associated with more radiation pneumonitis, 45% versus 30% for placebo, but no instances were grade 3 or higher. There were no adverse events leading to death; roughly 2% of patients in each arm discontinued treatment due to adverse events.
ILCN Editor Corey Langer, MD, said this trial joins the LAURA study in showing a clear cut, statistically significant and clinically meaningful PFS benefit for consolidation with a third-generation EGFR TKI following “standard” chemoradiation in patients with EGFRm LA-NSCLC. Osimertinib and aumolertinib share this distinction, he said.