In the era of precision medicine, molecular profiling of advanced lung cancers is essential for identifying the presence of genomic alterations to guide treatment selection. Two options are available to achieve this goal: direct testing of tumor tissue or testing cell-free DNA (cfDNA) from a liquid biopsy. But is one better than the other?
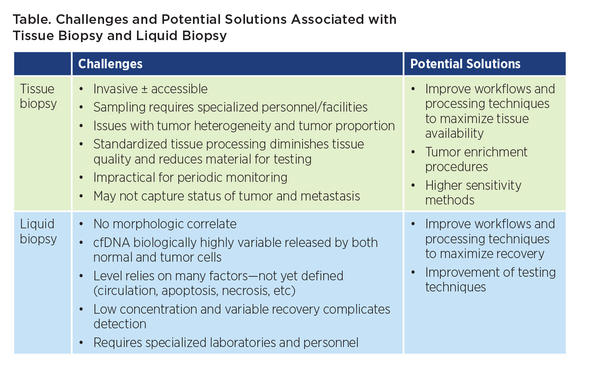
As discussed during a “crossfire session” pitting tissue biopsy against liquid biopsy, both options feature a number of advantages and disadvantages, according to presenters Marina Garassino, MD, of the National Cancer Institute of Milan, and Maria Arcila, MD, of the Memorial Sloan Kettering Cancer Center. For example, whereas liquid biopsy analysis can potentially overcome challenges posed by tumor heterogeneity within a tissue sample, this advantage is rendered moot if the tumor does not shed sufficient DNA into circulation (Table).
Studies have been performed to determine if one type of biopsy is superior to the other. A recent meta-analysis of 40 studies comparing the accuracy of liquid biopsy testing with that of tissue biopsy testing found that liquid biopsy performed just as well, if not better, than tissue biopsy for enabling the detection of EGFR mutations in patients with NSCLC.1 However, in patients with a negative liquid biopsy result, the lower sensitivity of the blood-based assay still necessitated tissue testing for confirmation of the lack of mutations.
It will be difficult for liquid biopsy to supplant tissue biopsy as the gold-standard source of material for molecular profiling at the time of diagnosis. IASLC guidelines currently dictate performing molecular profiling on a surgical specimen, preferably using next-generation sequencing (NGS) to capture all relevant alterations.2 However, one of the greatest challenges with any type of tissue specimen—be it a surgical specimen, tumor biopsy, or cytology sample—is obtaining sufficient high-quality material for NGS analysis.
Real-world Application
As a case in point, using routine tissue processing, data from the Memorial Sloan Kettering IMPACT study showed that approximately 25% of NSCLC tissue samples failed to yield NGS results.3 Biopsy samples and cytology specimens generated the highest failure rates, largely owing to insufficient DNA or tumor material. Improving workflows and processing techniques can help to overcome some technical challenges to provide high success rates but require extensive workflow and highly orchestrated institutional initiatives to implement.4,5 (ref). Alternative strategies are therefore needed that are easy to adopt broadly by any laboratory, whether academic or in the community.
In situations where insufficient tissue is available for analysis, Dr. Arcila thinks that liquid biopsy holds great value. It also prevails when a biopsy is not feasible or when timing is critical. For example, in case of delays in procuring tumor tissue for analysis, liquid biopsy testing offers a solution for quickly obtaining pertinent information. However, Dr. Arcila does not think this necessarily obviates the need for tissue analysis. “There is still very much value for that tissue,” she remarked during the live Q&A, at initial diagnosis, morphologic assessment is a pivotal component of the tumor classification and staging of the patient. Also, lack of shedding of tumor DNA into circulation in many tumors lead to negative results and the potential overlap of mutations in different tumor types outside of lung malignancies place limits of a liquid biopsy as a sole method of assessment.
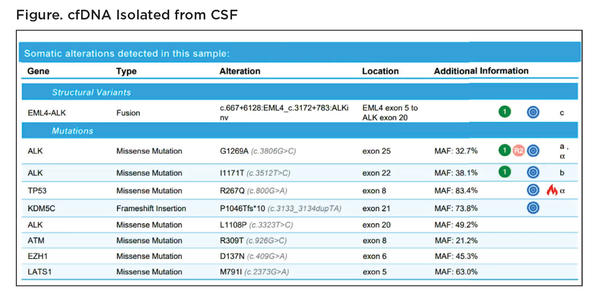
Still, there are some situations in which cfDNA analysis is clearly more informative than tissue analysis, for example, when trying to determine the mutational status of disease that has spread to the central nervous system (CNS). Dr. Acila described a patient case who originally presented with NSCLC featuring an EML4-ALK fusion. The patient responded to treatment but later developed CNS involvement. The primary tumor could no longer be biopsied, and cfDNA from plasma yielded indeterminate results. The only thing left to test was the CNS lesion, but it could not be biopsied based on its location. Instead, Dr. Acila’s team tested cfDNA in the cerebrospinal fluid (CSF). “The CSF not only shows the fusion that was originally diagnosed but also mutations in ALK that are associated with resistance that can guide further treatment decisions on this patient,” Dr. Acila showed (Figure). Notably, many of the CSF mutations were present at very high variant allele frequencies, much higher than could be detected in plasma cfDNA or in the primary tumor specimen, underscoring the valuable information obtained through the liquid CSF biopsy.
Finding the Optimal Solution
Overall, rather than viewing one method as superior to the other, both Dr. Garassino and Dr. Acila see tissue biopsy and liquid biopsy as complementary.
“Our goal is to fi nd the correct diagnosis for our patients and not to miss the targets that in 2020 can potentially be targetable,” Dr. Garassino explained. Using
the two methods in combination in select cases can help achieve that goal better than either method alone by increasing the overall sensitivity and specificity for
mutation detection.
“While testing a tissue sample with high tumor content upfront provides a much wider spectrum of profiling and is considered a more appropriate baseline to better assess not only the mutations but tumor mutation burden, microsatellite instability and copy number alterations (with a morphologic correlate)—the cfDNA, on the other hand, can capture tumor heterogeneity, multiple foci of the same tumor or the profi les of multiple tumors.
Thus, just as clinicians now tailor treatment for each patient based on genomic alterations, they may need to begin tailoring tumor testing methods—be it tissue biopsy, liquid biopsy, or both—based on patient-related factors, disease characteristics, and even the availability and allocation of institutional resources. “Only with the molecular tumor board together with the multidisciplinary team are you able to find the best personalized strategy for each patient,” emphasized Dr. Garassino.
References:
1. Wang N, Zhang X, Wang F, Zhang M, Sun B, Yin W, Deng S, Wan Y, Lu W. Th e diagnostic accuracy of liquid biopsy in EGFR-mutated NSCLC: a systematic review and meta-analysis of 40 studies. SLAS Technol. 2020:2472630320939565.
2. Rolfo C, Mack PC, Scagliotti GV, et al. Liquid biopsy for advanced non-small cell lung cancer (NSCLC): a statement paper from the IASLC. J Thorac Oncol. 2018;13(9):1248-1268.
3. Zehir A, Benayed R, Shah RH, et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat Med. 2017;23(6):703-713.





