In 2017, the promise of neoadjuvant immunotherapy in lung cancer led leaders from the IASLC and the US Food and Drug Administration to come together to discuss the opportunities in this space. Together with investigators from every specialty in thoracic oncology as well as regulators and pharmaceutical industry leaders, these stakeholders outlined what would be needed to capitalize on those opportunities to improve lung cancer care. And thus began a multi-year effort and series of projects spearheaded by the IASLC.

Mark G. Kris, MD, the William and Joy Ruane Chair in Thoracic Oncology at Memorial Sloan Kettering, New York, reviewed these initiatives and their implications at the 2024 Targeted Therapies of Lung Cancer meeting, which took place February 21-24 in Santa Monica, California. Dr. Kris leads the IASLC Pathologic Response Project.
“We listed a lot of needs and opportunities, and there were two needs that we saw that we could fill,” Dr. Kris said. “One was to develop rigorous methods to analyze the resection specimens to determine pathologic response, and the second—which is even more interesting and difficult—was to take pathologic complete response (pCR) and see if we could use that as an early clinical trial endpoint. Does path CR signify benefits in disease free and overall survival? It is a herculean task that no other disease has done.”
The work to analyze resection methods began straight away; and, under the leadership of William Travis, MD, Sanja Dacic, MD, and Ignacio Wistuba, MD, multidisciplinary recommendations for the pathologic assessment of lung cancer resection specimens following neoadjuvant therapy were published in 2020.1 The work continued with a study, which was published in October 2023, designed to test reproducibility among pathologists of pathologic response determinations using the 2020 recommendations.2
“Many of you were in Singapore last fall when Sanja Dacic presented the reproducibility data,” Dr. Kris said. “What they showed was there was a high degree of reproducibility in the way pathologists visually read these pathologic specimens.”
Next, an effort began to apply an AI-driven digital pathology assessment of pathologic response and compare this to manual assessments.
“Using the same specimens that were used for the reproducibility work, we will see if we can use digital pathology to, No. 1 get as good or better reproducibility, and No. 2 find a simpler way of doing this,” Dr. Kris said. “The pathologists that do this know this is very difficult. I think all of us see that path reports of the neoadjuvant specimens go on for pages. That’s a lot of work by our pathologic colleagues.”
Dr. Kris said he was also interested to see if the analysis of pCR by digital technology may be even better at correlating with event-free and overall survival. Researchers have a digital platform and AI algorithm to compare visual examination of major pathologic response to digital platforms.
“What you see is they are clearly comparable,” Dr. Kris said. “It keeps the hope alive that the digital reading might have greater ability to discriminate between long-term outcomes and pathologic response, and maybe to do it in a more efficient way. Can you envision that no matter where you are on earth, you could make digital images of your material and have this reviewed? And also have it less subject to variability?”
This work is expected to be completed by the end of the year, which may provide insight into such questions.
From there, Dr. Kris said the toughest task will be proving pCR is an early clinical trial endpoint.
“Think about what this would mean,” he said. “Think about what that does to the timelines of clinical trials… After perhaps 2 months of care, you could accurately predict long-term outcomes.”
However, sufficient data are needed to prove that pCR could be a justifiable early clinical trial endpoint, and Dr. Kris said this will require a group effort to compile that data.
“You need a lot of trials, not just a lot of data, and not any one country or any one company can do this,” he said. “So right now we are creating a database. Many are submitting phase 2 data in the neoadjuvant immunochemotherapy space, and all our pharma supporters with large trials have agreed to contribute their data.”
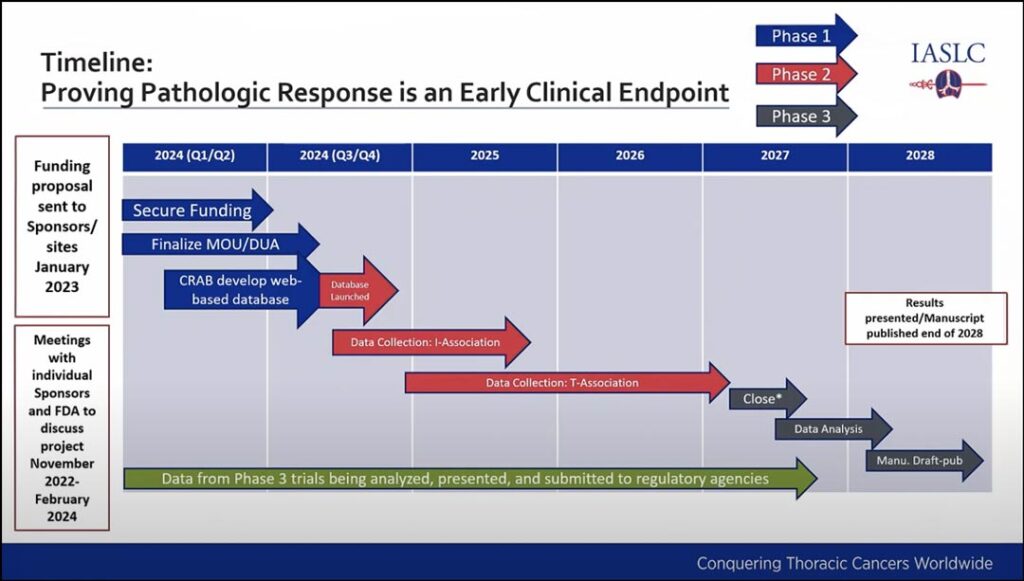
Researchers will be looking to see if pCR leads to long-term benefit and if pCR can be translated into treatment effect for EFS and PFS. The project will require a multi-year effort that is expected to be completed by 2028. (See Fig. 1)
While it is a large undertaking, Dr. Kris said he is confident it can be achieved.
References
- 1. Travis WD, Dacic S, Wistuba I, et al. IASLC Multidisciplinary Recommendations for Pathologic Assessment of Lung Cancer Resection Specimens After Neoadjuvant Therapy. J Thorac Oncol. 2020;15(5):709-740. doi:10.1016/j.jtho.2020.01.005
- 2. Dacic S, Travis W, Redman M, et al. International Association for the Study of Lung Cancer Study of Reproducibility in Assessment of Pathologic Response in Resected Lung Cancers After Neoadjuvant Therapy. J Thorac Oncol. 2023;18(10):1290-1302. doi:10.1016/j.jtho.2023.07.017





