-
Interim NADIM ADJUVANT Data Suggest Adjuvant Chemo-IO May Reduce Recurrence Risk in Patients Following Complete Resection
-
Final FLAURA2 OS Data Show Osimertinib Plus Chemo Offers Benefit Compared with Monotherapy
-
Potential Impact of GLP1-RAs on TKI-induced Weight Gain in Patients with NSCLC
-
No “Known” Risk Factors: The Health Consequences of Radiation Therapy
-
Discussion Covered Updates, Strategies, and Controversies in Lung Cancer Staging
-
No PFS, OS Benefit Seen in Final Results from PACIFIC-2
-
WCLC Attendees Hear Preview of Proposed Changes for the 9th Edition of the TNM Staging Classification for Thoracic Cancers
-
Treatment of Immunotherapy-Related Dermatologic Toxicities: An Interview with Dr. Mario Lacouture
-
A Holistic Approach to Patient Care
Industry News & Regulatory Approvals
-
First Comprehensive Genomic Profiling Assay Approved for Use with Brigatinib, ALK-positive NSCLC
July 1, 2021—The U.S. Food and Drug Administration (FDA) has approved FoundationOne CDx for use as a companion diagnostic for brigatinib, which the FDA had previously approved for treatment of […]
-
Selpercatinib Approved in Canada for Metastatic NSCLC with RET Fusions
June 15, 2021—Canada’s medical regulatory agency approved the use of selpercatinib monotherapy for treatment of patients with metastatic RET fusion–positive NSCLC. Health Canada based its conditional approval on the LIBRETTO-001 […]
-
Sotorasib Wins Approval for Unmet Need in KRAS G12C-Driven NSCLC
On May 28, the US Food and Drug Administration (FDA) granted accelerated approval of sotorasib for patients with KRAS G12C-mutated NSCLC who have received at least one prior line of […]
-
Tepotinib Approved for MET Exon 14 Skipping Mutated Metastatic NSCLC
February 3, 2021—Tepotinib was approved for treatment of patients with metastatic NSCLC who harbor MET exon 14 skipping alterations. Approval was based on data from the VISION trial (NCT02864992). VISION, […]
-
Osimertinib Approved as Adjuvant Therapy for NSCLC with Certain EGFR Mutations
December 18, 2020—The U.S. Food and Drug Administration (FDA) approved osimertinib as post-resection adjuvant therapy for patients with NSCLC and EGFR exon 19 deletions or exon 21 L858R mutations, as […]
-
First Therapeutic Approval in 16 years for MPM
October 2, 2020—As part of the U.S. Food and Drug Administration’s (FDA) Project Orbis, the FDA approved combination nivolumab and ipilimumab for treatment-naive patients with unresectable malignant plueral mesothelioma (MPM). […]
-
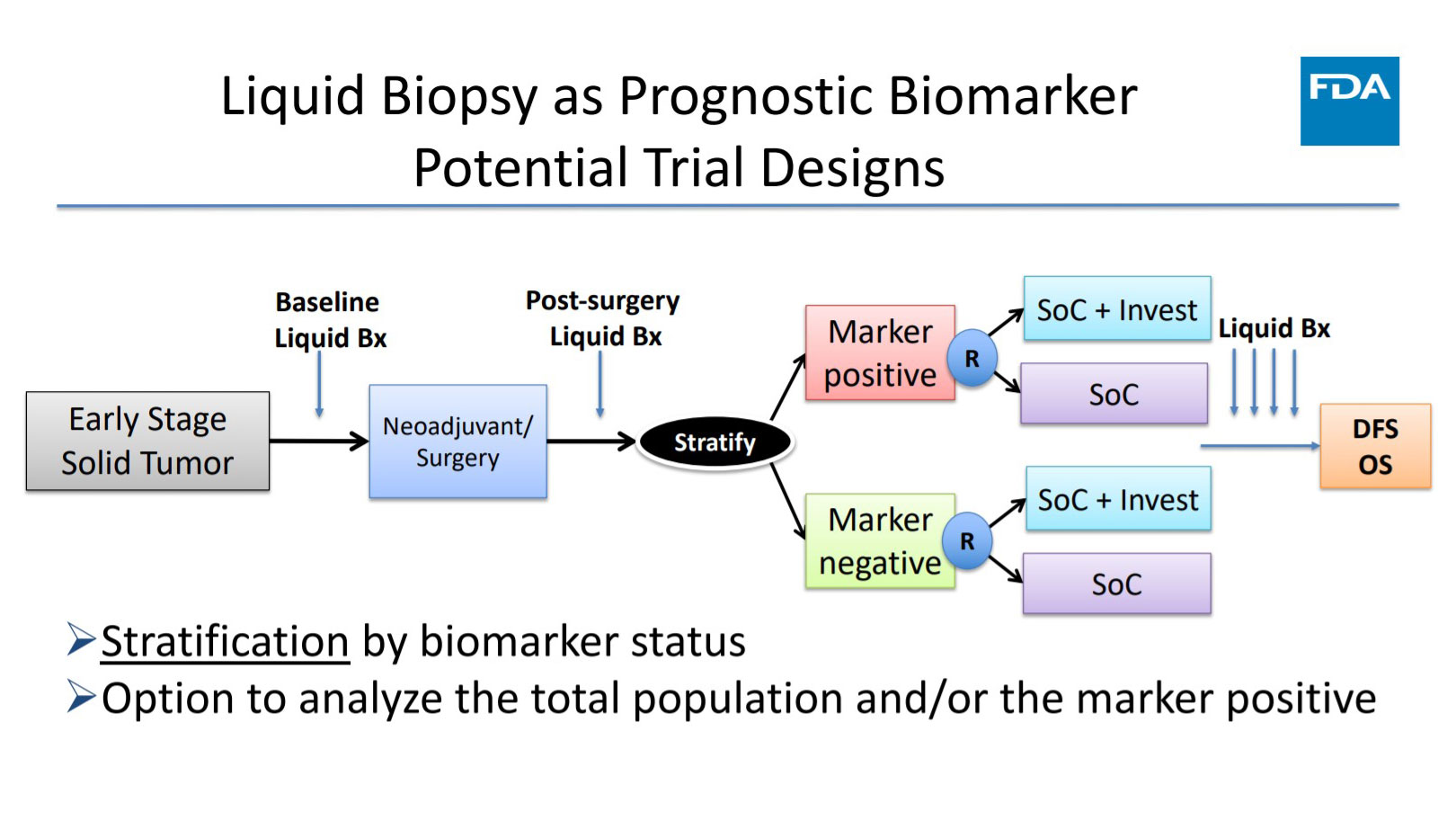
FDA Perspectives on the Use of Liquid Biopsy in NSCLC
The US Food and Drug Administration (FDA) recognizes the value of liquid biopsy in managing patients with cancer, and the agency is actively working to bring these assays to the clinic. Harpreet Singh, MD, Director of […]
-
Funding Opportunities for Innovative Multidisciplinary Cancer Research Teams
The US National Cancer Institute (NCI), a division of the US National institutes of Health (NIH), is partnering with Cancer Research UK, the world’s largest independent cancer research charity, to […]
-
FDA Approves Pralsetinib for RET Fusion–Positive NSCLC
The US Food and Drug Administration (FDA) granted Accelerated Approval to pralsetinib as treatment for adults with metastatic NSCLC who harbor RET fusions. The first and only test to identify RET fusion–positive NSCLC, the Oncomine Dx Target […]
-
Huge Strides for Liquid Biopsy Assays with Two FDA Approvals
August 2020 was a groundbreaking month for liquid biopsy technology, with two assays approved by the US Food and Drug Administration (FDA) as companion diagnostics just weeks apart. The Guardant360 CDx assay, approved August 7, was […]