On May 28, the US Food and Drug Administration (FDA) granted accelerated approval of sotorasib for patients with KRAS G12C-mutated NSCLC who have received at least one prior line of […] Read more
February 3, 2021—Tepotinib was approved for treatment of patients with metastatic NSCLC who harbor MET exon 14 skipping alterations. Approval was based on data from the VISION trial (NCT02864992). VISION, […] Read more
December 18, 2020—The U.S. Food and Drug Administration (FDA) approved osimertinib as post-resection adjuvant therapy for patients with NSCLC and EGFR exon 19 deletions or exon 21 L858R mutations, as […] Read more
October 2, 2020—As part of the U.S. Food and Drug Administration’s (FDA) Project Orbis, the FDA approved combination nivolumab and ipilimumab for treatment-naive patients with unresectable malignant plueral mesothelioma (MPM). […] Read more
FDA Perspectives on the Use of Liquid Biopsy in NSCLC
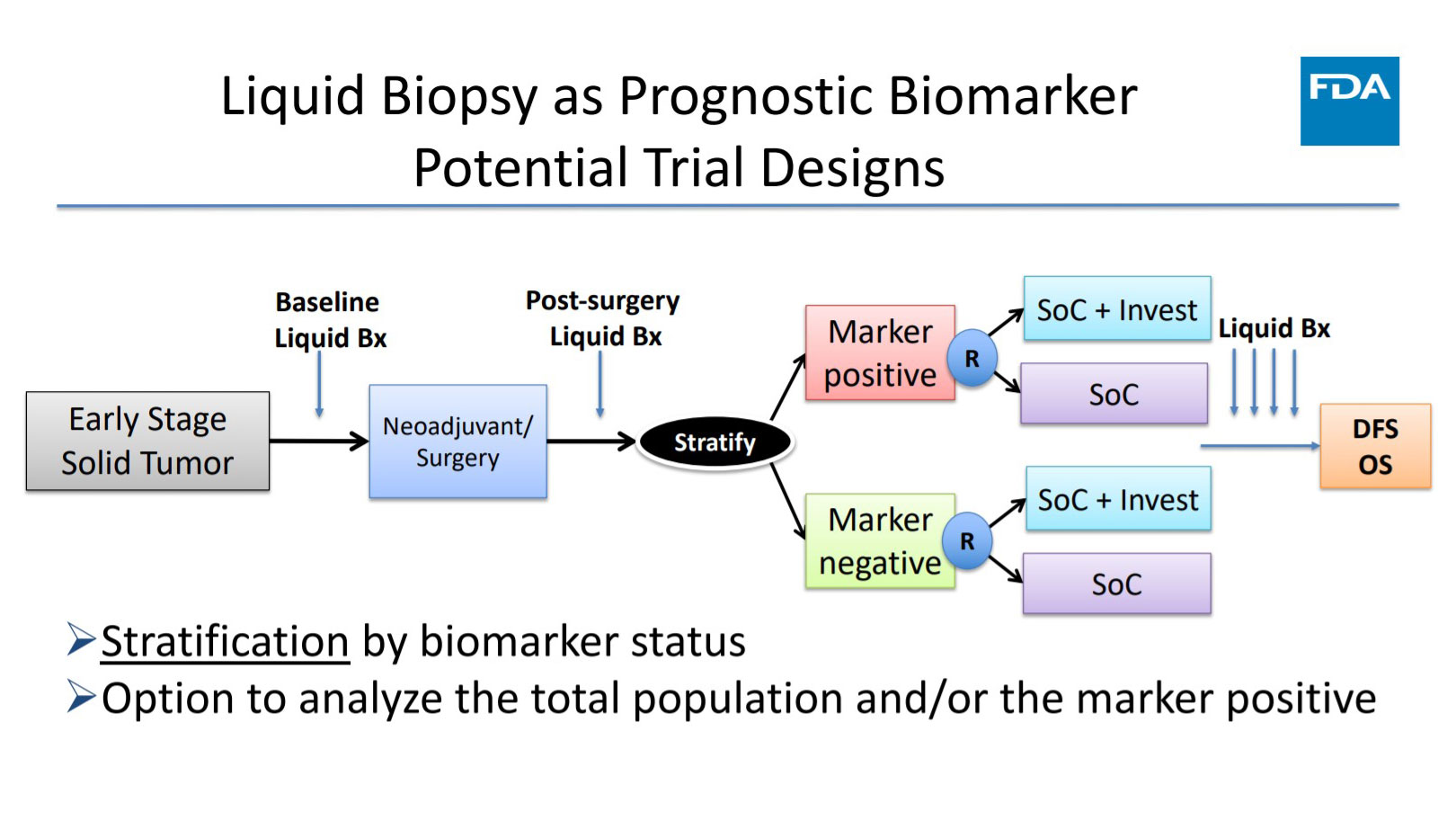
Kara Nyberg, PhDThe US Food and Drug Administration (FDA) recognizes the value of liquid biopsy in managing patients with cancer, and the agency is actively working to bring these assays to the clinic. Harpreet Singh, MD, Director of […] Read more
The US National Cancer Institute (NCI), a division of the US National institutes of Health (NIH), is partnering with Cancer Research UK, the world’s largest independent cancer research charity, to […] Read more
The US Food and Drug Administration (FDA) granted Accelerated Approval to pralsetinib as treatment for adults with metastatic NSCLC who harbor RET fusions. The first and only test to identify RET fusion–positive NSCLC, the Oncomine Dx Target […] Read more
August 2020 was a groundbreaking month for liquid biopsy technology, with two assays approved by the US Food and Drug Administration (FDA) as companion diagnostics just weeks apart. The Guardant360 CDx assay, approved August 7, was […] Read more
Dr. Harpreet Singh Discusses the Conduct of FDA Clinical Trials During the COVID-19 Pandemic
Harpreet Singh, MDHarpreet Singh, MD, is director of Division of Oncology 2 at the U.S. Food and Drug Administration (FDA). In the following interview, she discusses the FDA’s response to the COVID-19 […] Read more
By Leah Lawrence Posted: June 24, 2020 As part of its declaration on tobacco cessation after cancer diagnosis, the IASLC recommended that smoking status, both initially and during the study, […] Read more