According to results of the CROWN study, patients with previously untreated, advanced ALK-positive non-small cell lung cancer (NSCLC) receiving lorlatinib, an ALK tyrosine kinase inhibitor (TKI), had longer progression-free survival (PFS) and better control and prevention of brain metastases compared to those treated with the first-generation ALK TKI crizotinib. The results of a 5-year analysis of the study were presented by Benjamin Solomon, MBBS, FRACP, PhD, on May 31 during the 2024 American Society of Clinical Oncology Annual Meeting.

The phase III CROWN study compares first-line treatment with lorlatinib to crizotinib in patients with previously untreated advanced ALK-positive non-small cell lung cancer
Dr. Solomon, a clinician-scientist at Peter MacCallum Cancer Center in Victoria, Australia, said the PFS observed with first-line lorlatinib is the longest ever reported in ALK-positive lung cancer. In fact, to his knowledge, it is the longest PFS seen with any targeted therapy in advanced NSCLC, he said. As of October 31, 2023—the date of the data lock for the analysis Dr. Solomon presented—the median progression-free survival (PFS) with lorlatinib had still not been reached.
“These systemic efficacy results coupled with protection from intracranial progression and the absence of new safety signals, indicates that first-line lorlatinib provides an unprecedented improvement in outcomes for patients with advanced ALK-positive non-small cell lung cancer,” Dr. Solomon said to applause from the audience.
Patients in the CROWN study were randomized 1:1 to lorlatinib or crizotinib. The primary end point of the study was PFS by blinded independent central review. Positive data from a previously presented interim analysis of CROWN formed the basis for global regulatory approvals for lorlatinib as a first-line treatment for advanced ALK-positive NSCLC. The new data presented by Dr. Solomon further support those approvals.
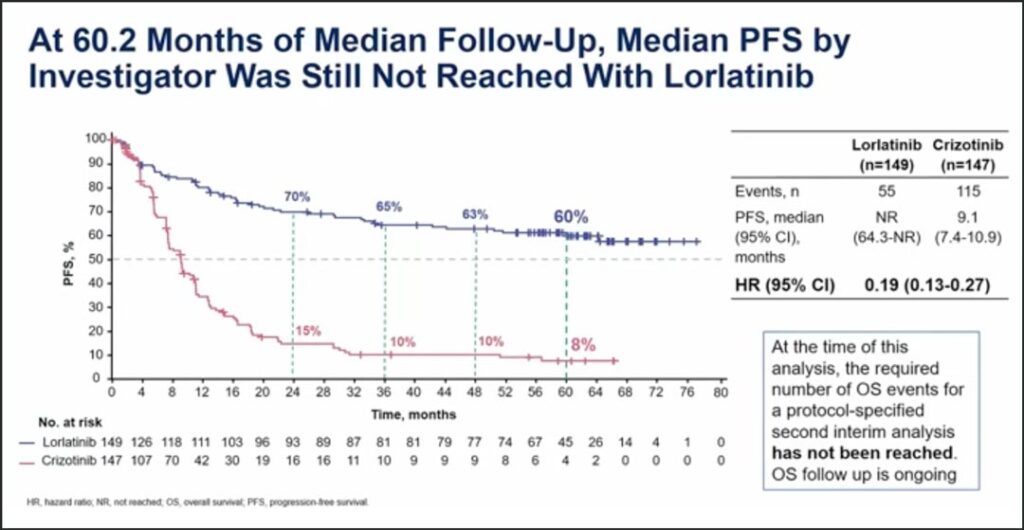
“After 60.2 months of median follow-up, the hazard ratio of lorlatinib versus crizotinib for progression-free survival was 0.19,” Dr. Solomon said. “The median progression-free survival with crizotinib was 9.1 months. At the landmark of 60 months, or 5 years, the progression-free survival with lorlatinib was 60% compared to 8% with crizotinib.” (See Fig. 1)
At the time of this analysis, the required number of events to trigger a protocol-specified analysis for overall survival had not been reached, so overall survival follow-up is ongoing.
Dr. Solomon said benefit with lorlatinib was observed across all subgroups and was seen irrespective of the presence of brain metastases, ethnic origin, sex, age, or smoking status.
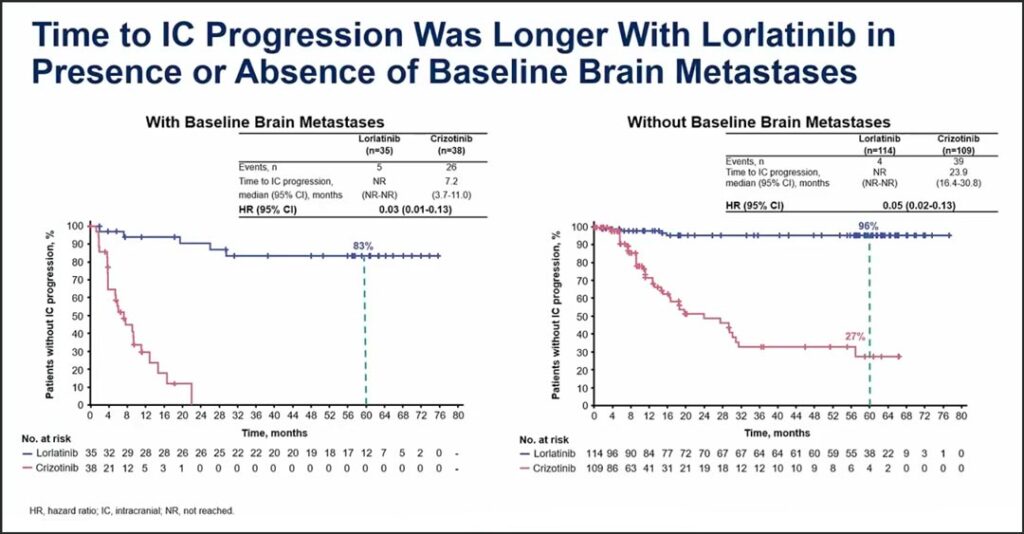
In his presentation, Dr. Solomon indicated that an important feature of the study is that baseline MRI scans were performed and were conducted every 8 weeks thereafter, allowing for assessment of intracranial efficacy measures, including time to CNS progression. Baseline brain metastases were present in about a quarter of patients and PFS benefit was seen both in patients with baseline brain metastases and in patients without baseline brain metastases.
In patients with baseline brain metastases, the hazard ratio was 0.08. In patients without brain metastases the hazard ratio was 0.24. In both groups, the median PFS was not reached with lorlatinib. For the intent-to-treat population, the hazard ratio for the time-to-intracranial progression was 0.06. The median time-to-intracranial progression with lorlatinib was not reached, with a 92% probability of being free of intracranial progression at 60 months, Dr. Solomon said.
“The CROWN results speak to the ability of lorlatinib not only to prevent the progression of existing brain metastases, but also to prevent the development of new brain metastases,” he said. (See Fig. 2)
According to the data, the safety profile was consistent with findings reported in the prior analysis; no additional safety concerns arose with additional follow up.
Grade 3-4 adverse events were seen in 77% of patients treated with lorlatinib and 57% of patients treated with crizotinib. The higher incidence of grade 3-4 adverse events with lorlatinib was largely attributed to elevated triglycerides and cholesterol as well as weight gain and hypertension. Dr. Solomon said CNS adverse events occurred in 42% of patients and were typically low grade. Additionally, treatment-related adverse events leading to permanent discontinuation of lorlatinib occurred in 5% of patients. All of these were reported during the first 26 months of treatment, he said.
Finally, Dr. Solomon shared data on the efficacy of lorlatinib compared with crizotinib by molecular subgroups that have been associated with poorer prognosis, such as EML4-ALK variant 3 (EMLR-ALK V3) and tumors with co-mutations in TP53. According to the analysis, in tumors with EMLR-ALK V3, the median PFS with lorlatinib was 60 months, and in patients with TP53 co-mutations the median PFS was 51.6 months, indicating a benefit in both of these molecular groups.
In contextualizing the results of CROWN, discussant Jessica Lin, MD, Associate Professor of Medicine at Harvard Medical School and Attending Physician at Massachusetts General Hospital Center for Thoracic Cancers and Henri and Belinda Termeer Center for Targeted Therapies, said the findings affirm lorlatinib as standard-of-care first-line therapy for patients with metastatic ALK-positive lung cancer.
“Using the best therapy up front remains imperative, especially given that a significant proportion of patients who receive a second-generation ALK inhibitor up front will not then go on to receive subsequent therapy at disease progression,” she said. “So, what will I do next week if a patient presents with a new diagnosis of metastatic ALK-positive lung cancer? Recognizing that treatment decisions will always need to be individualized, lorlatinib will be my preferred initial therapy.”