One reason lung cancer is the oncologic disease with the highest incidence and mortality rate worldwide is the 30% to 45% 5-year relapse risk in patients who have undergone surgery for early-stage disease with curative intent (Abstract MA08.05).
Those numbers are driving researchers to consider whether some patients should receive adjuvant treatment to reduce their risk of relapse, and, if so, how to identify the individuals most likely to benefit. It is an active area of investigation, with numerous studies considering proposed adjuvant treatment regimens such as novel tyrosine kinase inhibitors (TKIs), and others testing predictive models focused on strategies such as next-generation sequencing or the detection of circulating-tumor DNA.
A milestone moment came in May 2020, when data from the ADAURA trial1 showed that adjuvant treatment using the TKI osimertinib improves disease-free survival in patients with stage IB to IIIA EGFR-mutated NSCLC. Yet, the study did not shed light on whether patients in this population with even earlier-stage disease might benefit from treatment.
Corey J. Langer, MD, a professor of medicine and director of thoracic oncology at Penn Medicine in Philadelphia, said he is “on the fence” about giving osimertinib to patients with stage IB or earlier disease because it causes chronic, low-grade side effects.
“It affects people’s quality of life across 3 years of treatment,” Dr. Langer, who is also the Editor of ILCN, said. “Before we start exposing people to therapy, I think it’s absolutely essential that we identify those expected to do poorly after resection and those who are intrinsically going to do well.”
The 2021 World Conference on Lung Cancer included a number of studies that sought to do just that by validating prognostic models in patients with early-stage NSCLC. The models incorporated factors including the results of whole-exon and transcriptome sequencing and disease characteristics such as pathologic stage, histologic subtype, tumor size, and the presence of lymphovascular invasion.
For example, a retrospective study conducted by Junji Ichinose, MD, PhD, and colleagues (Abstract MA08.01) considered whether the presence of a high-grade (solid or micropapillary) NSCLC subtype in patients with EGFR-driven NSCLC — whether predominant or not — could be considered an independent predictor of the recurrence of stage I lung adenocarcinoma.
Study Details
The researchers found that high-grade histology in this population, even if not predominant, was an independent predictor of poorer recurrence-free survival (RFS) (hazard ratio = 1.838 [1.149-2.939], p = 0.011). However, in those who had EGFR wild-type disease, high-grade subtype was not a predictor of recurrence.
“Histological subtypes, including minor components, should be considered when evaluating the risk of recurrence in patients with EGFR-mutated lung adenocarcinoma,” Dr. Ichinose, of Cancer Institute Hospital JFCR in Tokyo, said in presenting the findings.
The investigators embarked on the study to build on previous evidence demonstrating that the EGFR mutation is a risk factor for disease recurrence when high-grade subtype is predominant.
They reviewed the records of 721 patients who had undergone both EGFR mutation analysis and curative surgery for their stage I disease. The team evaluated the OS and RFS of participants according to EGFR mutation status and the presence of a high-grade subtype component of greater than 5%. Additional multivariable analysis factors included age of 70 years or older, gender, smoking habits, invasion size greater than 10 mm, pleural invasion, lymphatic invasion, and vascular invasion.
Of all participants, 375 (52%) had EGFR-positive disease, which was associated with female gender, nonsmoking status, lower consolidation/tumor ratio, absence of high-grade subtype, and absence of lymphovascular invasion.
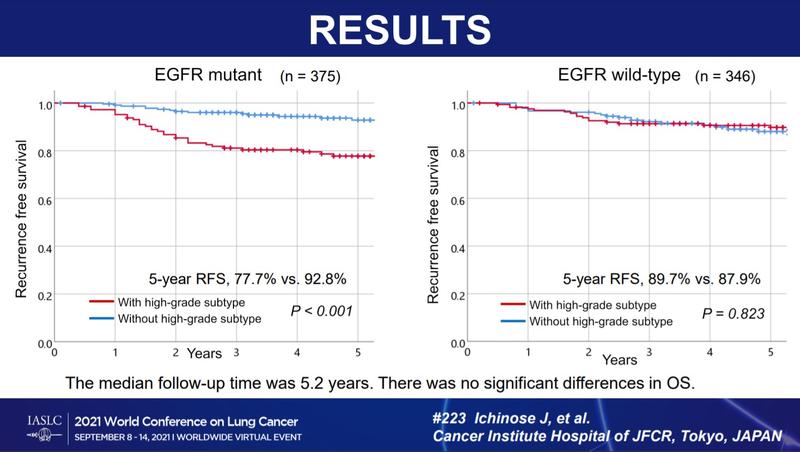
After a median follow-up of 5.2 years, relapse was observed in 57 (7.9%) cases, with 21 patients (2.9%) dying due to relapse and 33 (4.6%) dying from other causes (Fig. 1). Patients with a high-grade disease subtype that was characterized by EGFR mutations experienced poorer RFS (5-year RFS 77.7% vs. 92.8%, p < 0.001). There was no significant prognostic difference for patients who did not have EGFR mutations in groups with versus without a high-grade subtype (5-year RFS 89.7% vs. 87.9%, p = 0.823).
There were no significant differences in OS between any of the study groups, Dr. Ichinose said.
“Multivariate analysis found that the combination of EGFR and the presence of a high-grade subtype was an independent predictive factor for pleural RFS…as well as pleural invasion and lymphovascular invasion, but it was not predictive of overall survival,” he said.
Considering the Implications
Discussant Els Wauters, MD, PhD, a professor at University Hospitals of Leuven in Belgium, noted that the components in the team’s predictive model matched a 2015 classification of stage 1 invasive adenocarcinoma by the World Health Organization. Under that guideline, patients with tumors that were predominantly micropapillary or solid were classified as having “high-grade” disease consistently associated with the worst prognoses.
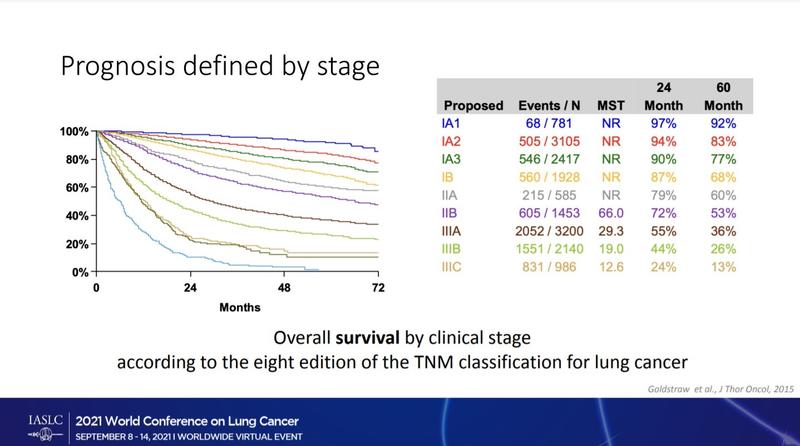
Dr. Wauters agreed with the study’s authors that their findings suggest a benefit of combining information on pathologic stage, histologic subtype, and EGFR status to help predict recurrence in patients with early-stage disease who have undergone complete resection (Fig. 2).
She added that, while testing for mutations such as EGFR is not yet the standard of care for early-stage disease, there is a rising interest in the practice in this setting. In particular, she said, the results of the ADAURA trial will lead to more molecular testing in early disease to ensure that eligible patients have the option of receiving treatment with osimertinib.
Patient advocate and lung cancer survivor Ivy Elkins also considers the data shared by Dr. Ichinose promising.
“It shows that there may be a way to stratify which patients have more of a likelihood of their disease coming back and should get further treatment,” she said. “Having that type of conversation with patients is becoming more and more important. Patients shouldn’t simply be told: ‘You had stage I disease, you were treated, and now you’re cured.’ The discussion needs to be more nuanced and more descriptive of the chance of recurrence, because patients don’t always realize that might happen, and they also want to balance the risks and benefits of potential treatment.”
Dr. Wauters cautioned that more research is needed on the predictive model, “since these findings were generated in small Japanese populations with a higher frequency of EGFR mutations and also a different adjuvant approach” compared with the United States.
Importantly, because the findings shared by Dr. Ichinose and his colleagues did not distinguish between patients with stage IA and IB disease, their study did not elucidate whether patients with stage IA NSCLC might benefit from treatment with osimertinib, and that will warrant further investigation, Dr. Langer said.
Reference
Astrazeneca.com. Phase III ADAURA trial showed treatment with Tagrisso after surgery with curative intent reduced the risk of disease recurrence or death by c. 80%. Published May 28, 2020. Accessed October 12, 2021. https://www.astrazeneca.com/media-centre/press-releases/2020/tagrisso-d…