There is a growing demand for targeted therapies in patients with ROS1-rearranged non-small cell lung cancer (NSCLC); However, some of the currently approved treatments have been associated with adverse side effects, including inadequate intracranial activity or central nervous system (CNS)-related toxicities.1
China’s National Medical Products Administration (NMPA) has recently accepted a marketing authorization application for foritinib, a novel ALK and ROS1 inhibitor. This decision is based on interim results from the phase III REMARK trial, which evaluated the safety and efficacy of foritinib for the treatment of advanced ROS1-rearranged NSCLC.1 The highly potent drug has also completed phase II trials in China for the treatment of ROS1-positive NSCLC.1
The interim results were presented during the 2024 World Conference on Lung Cancer (WCLC). Registered IASLC members can view the on-demand session on Lung Cancer 360.
Of the 275 enrolled participants, 139 were administered a daily 160-mg dose of foritinib, while 136 participants received a 250-mg dose of crizotinib twice daily. The data indicate a promising efficacy for foritinib, with significant improvements in progression-free survival (PFS) compared with the crizotinib cohort. According to the findings, participants in the crizotinib cohort had a median PFS of 13.93 months, while the median PFS for those in the foritinib group was not reached (NR).1

Foritinib also demonstrated significant reductions in the risk of CNS progression compared with crizotinib, with the median CNS time to progression (CNS-TTP) not reached for foritinib versus 19.32 months for crizotinib.1 The objective response rate (ORR) results also favored the foritinib cohort, showing an ORR of 92.8% compared with 80.9% for participants treated with crizotinib.1
Overall survival (OS) was also improved with foritinib treatment versus crizotinib. According to the data, the median OS was not reached in participants treated with foritinib, while it was 24.94 months for the crizotinib cohort.
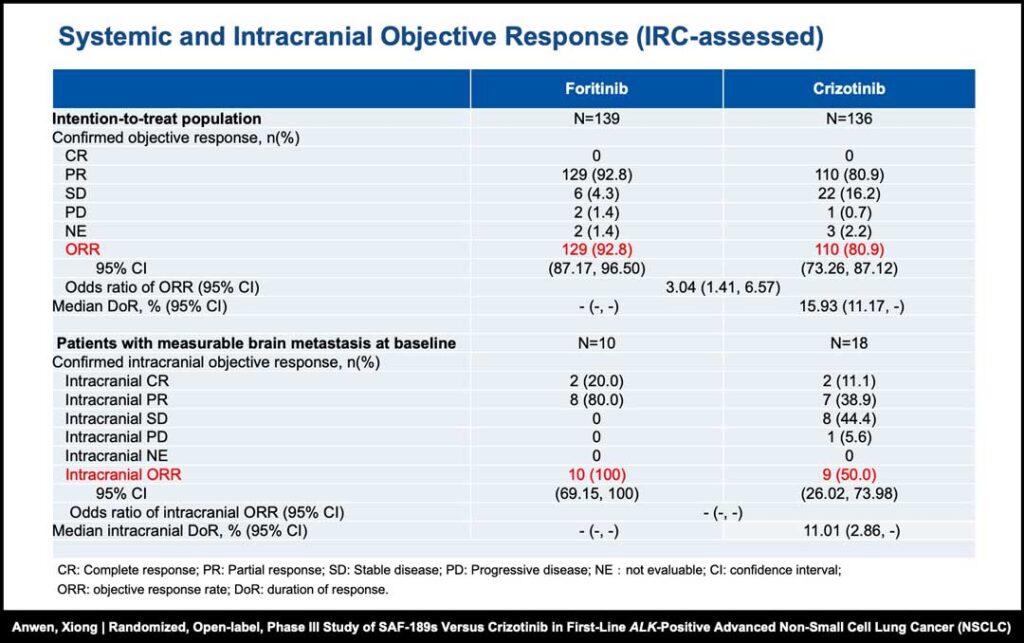
Furthermore, all participants with baseline brain metastases demonstrated an intracranial ORR in the foritinib group (100%), compared with only 50% in the crizotinib group.1 The foritinib group also exhibited fewer incidences of grade 3 and 4 treatment-related adverse events (TRAEs) compared with crizotinib (37.7% vs. 55.6%).1

Hyperglycemia and hypertension were the most common grade 3 or higher TRAEs in the foritinib group. Compared with the crizotinib cohort, those treated with foritinib experienced fewer TRAEs that resulted in dose interruptions (26.8% vs. 35.6%) or dose reductions (23.9% vs. 37.8%).1
Overall, the results of the REMARK trial indicate that foritinib demonstrated improved PFS and a reduced risk of CNS progression compared with crizotinib. Additionally, no new safety signals were identified following treatment with foritinib. If approved, foritinib could open the door for a new therapeutic option for patients with NSCLC and help address the current challenges in treating ALK-positive NSCLC.
References










