Treatment guidelines recommend neoadjuvant or adjuvant therapies with surgery for patients with large primary tumors or clinical evidence of intrathoracic nodal spread.1,2 A recent joint IASLC–U.S. Food and Drug Administration workshop identified many advantages to the neoadjuvant approach.3 Pathologic response after neoadjuvant therapies in resection specimens can serve as a surrogate endpoint for PFS and OS in lung cancer.4 In contrast to the detailed recommendations for pathology processing of breast carcinoma resection specimens, osteosarcoma, and colorectal and esophageal carcinomas, few studies describe approaches for gross processing and microscopic assessment of lung resection specimens following neoadjuvant therapies.
An IASLC multidisciplinary panel led by Pathology Committee members and assisted by experts in thoracic medical oncology, thoracic surgical oncology, and radiology recognized this practice gap; and took on the task of reviewing published literature and writing recommendations for pathologic assessment of resected lung cancer specimens following neoadjuvant therapies. Their report was reviewed by the entire IASLC Pathology Committee and the membership of the Pulmonary Pathology Society before it was distributed electronically by the IASLC office for public comments. The document was also reviewed by the U.S. Food and Drug Administration (G. Blumenthal); the European Medicines Agency, The Netherlands (R. Herold); the Office of New Drug, Pharmaceuticals and Medical Devices Agency, Japan (K. Kiyohara); and the Office of Clinical Evaluation, Center for Drug Evaluation, National Medical Products Administration, P.R. China (Z. Yang). The full report was published in the Journal of Thoracic Oncology.5
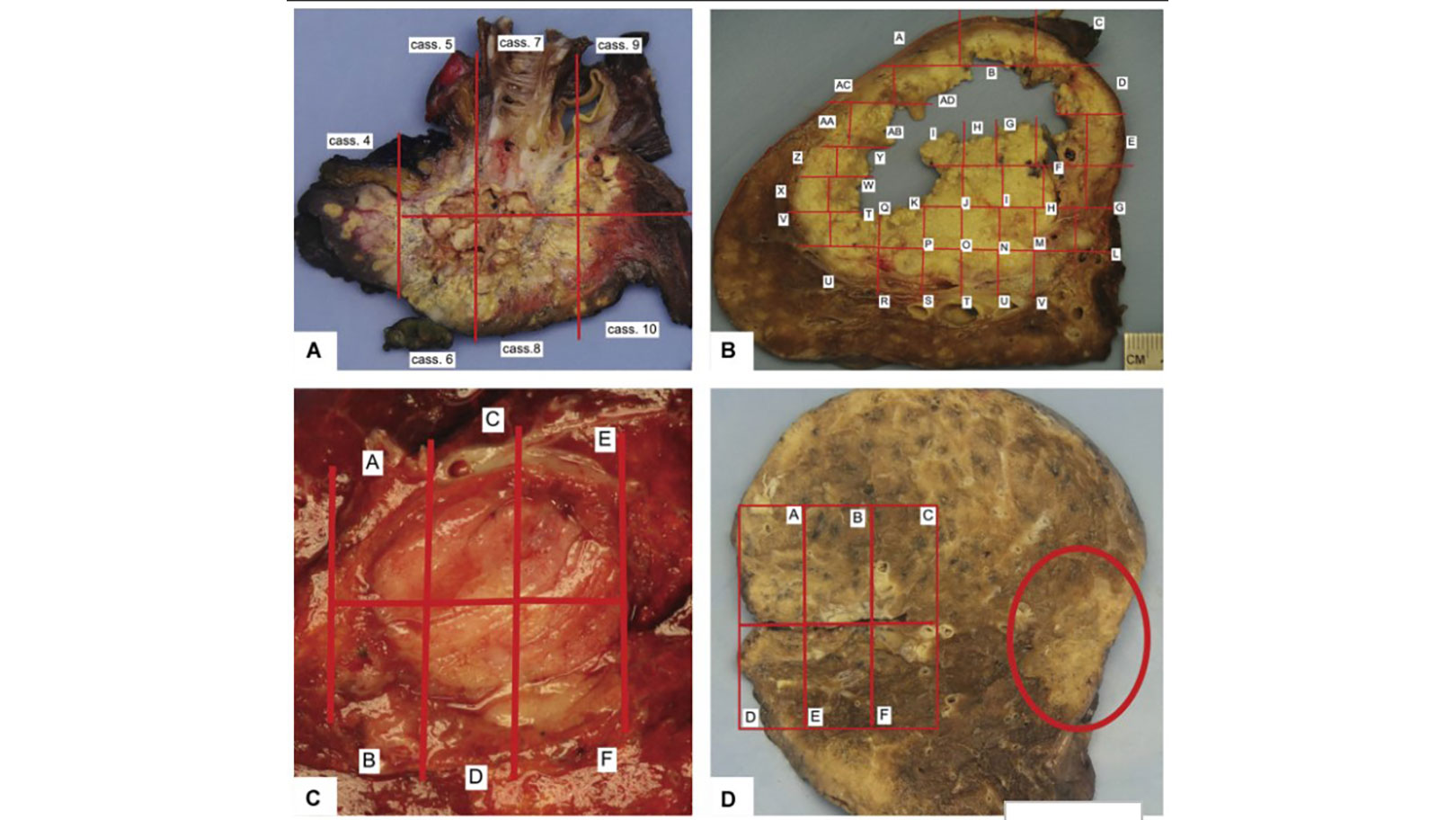
The IASLC guideline details a standardized approach to gross processing of lung cancer resection specimens irrespective of the type of neoadjuvant treatment—chemotherapy, chemoradiation, targeted therapies, or immunotherapies (Fig. 1). The document also provides definitions of major pathologic response (MPR) and complete pathologic response (CPR). The historical definition of MPR for all histologic types of lung cancers—10% or less viable tumor—–has been adopted in the current College of American Pathologists synoptic reports for primary lung cancers and by the Royal College of Pathologists. The 10% or less cutoff has been recently challenged by new data: A review of 272 resection specimens suggested that the threshold rate of viable tumor for MPR may be different for adenocarcinomas (65% cutoff) and squamous cell carcinomas (10% cutoff).6 However, the IASLC panel recognized that additional validation studies are needed before different cutoff points can be recommended on the basis of histologic type of the tumor or the type of neoadjuvant therapy administered. The IASLC guideline proposal includes an updated, detailed synoptic template for reporting pathologic findings in neoadjuvant cases that we hope will be adopted by the College of American Pathologists and used by pathologists worldwide.

A simple and uniform approach is recommended for microscopic evaluation of the area where the original pretreatment tumor was located (i.e., the tumor bed). It suggests reporting and quantification of the major components of the specimen: viable tumor, necrosis, and stroma (which can include fibrosis and inflammation; Fig. 2). Estimations of the three components should add up to 100%. The panel noted that the same histologic changes can be seen in tumors from patients who did not receive neoadjuvant therapy; however, none achieved MPR or CPR without induction therapy.7 Therefore, it is essential that surgeons communicate the treatment history to pathologists at the time of intraoperative consultation and/or on the requisition form. Otherwise, these changes may be considered to represent native tumor features rather than response to neoadjuvant therapies.
In this proposal, the term “tumor bed” is preferred rather than “regression bed,” which has been introduced for neoadjuvant immunotherapy.8 Tumors treated with checkpoint inhibitors can show features considered to reflect immune activation such as proliferative fibrosis, neovascularization, cholesterol clefts, high number of tumor-infiltrating lymphocytes, and tertiary lymphoid structures. Cottrell and colleagues reviewed 20 resection specimens following neoadjuvant nivolumab, and they proposed immune-related pathologic response criteria (irPRC), for which percent response is calculated as residual viable tumor (RVT)/tumor bed, where tumor bed includes RVT plus regression bed plus necrosis. These criteria have also been used in the proposed Pan-Tumor pathologic scoring system. The morphologic features of the regression bed as proposed for immunotherapies are also applicable to stroma of tumors treated with other types of neoadjuvant therapies and, therefore, are included in the IASLC proposal for stromal features. However, the stroma may have other appearances that are more like native tumor stroma, and these are included in the proposed criteria as well. The panel felt that pathologists, including specialists in thoracic pathology, cannot reliably distinguish these features in every case. The proposed immune scoring system includes intratumoral stroma as residual viable tumor in cases without features of regression. This is in contrast to the IASLC proposal that recommends including only viable tumor cells as residual viable tumor, whereas intratumoral stroma is considered stroma.
Today, we don’t know if different scoring systems will be needed for different types of neoadjuvant therapies. No studies or series directly compare the predictive value of these two systems. Furthermore, there are very limited data about the diagnostic reproducibility of the proposed criteria, and those that do exist come from a very limited number of cases and only a few pathologists. The IASLC is initiating a study of a large number of resected cases with and without neoadjuvant therapies to address diagnostic reproducibility and the predictive value of the proposed scoring systems.
The guideline also provides recommendations for questions that are not well addressed in the literature. These include the assessment of cases with multiple tumor nodules of invasive carcinomas, multiple adenocarcinomas in situ and/or minimally invasive adenocarcinomas, and colloid adenocarcinomas. Similarly, limited or no data exist for the assessment of response in lymph nodes or resected distant sites with metastatic carcinomas. The recommendations were based on expert opinion and practices today employed by pathologists evaluating other primary sites (i.e., gastrointestinal tract and pancreas for colloid adenocarcinoma). The IASLC recommendations are the first attempt to standardize pathology practices in the assessment of lung cancer resection specimens after neoadjuvant therapy. The application of these recommendations will provide direction for future studies that will validate and/or lead to the modification of these proposals.
Figures.
Reprinted with permission from Journal of Thoracic Oncology.
References:
1. Ettinger DS, Wood DE, Aggarwal C, et al. NCCN guidelines insights: non-small cell lung cancer, Version 1.2020. J Natl Compr Canc Netw. 2019;17(12):1464-1472.
2. Kris MG, Gaspar LE, Chaft JE, et al. Adjuvant systemic therapy and adjuvant radiation therapy for stage I to IIIA completely resected non-small-cell lung cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice guideline update. J Clin Oncol. 2017;35(25):2960-2974.
3. Blumenthal GM, Bunn PA Jr, Chaft JE, et al. Current status and future perspectives on neoadjuvant therapy in lung cancer. J Thorac Oncol. 2018;13(12):1818-1831.
4. Hellmann MD, Chaft JE, William WN Jr, et al. Pathological response after neoadjuvant chemotherapy in resectable non-small-cell lung cancers: proposal for the use of major pathological response as a surrogate endpoint. Lancet Oncol. 2014;15(1):e42-50.
5. Travis WD, Dacic S, Wistuba I, et al. IASLC multidisplinary recommendations for pathologic assessment of lung cancer resection specimens following neoadjuvant therapy. J Thorac Oncol. 2020;15(5):709-740..
6. Qu Y, Emoto K, Eguchi T, et al. Pathologic assessment after neoadjuvant chemotherapy for NSCLC: importance and implications of distinguishing adenocarcinoma from squamous cell carcinoma. J Thorac Oncol. 2019;14(3):482-493.
7. Pataer A, Kalhor N, Correa AM, et al. Histopathologic response criteria predict survival of patients with resected lung cancer after neoadjuvant chemotherapy. J Thorac Oncol. 2012;7(5):825-832.
8. Cottrell TR, Thompson ED, Forde PM, et al. Pathologic features of response to neoadjuvant anti-PD-1 in resected non-small-cell lung carcinoma: a proposal for quantitative immune-related pathologic response criteria (irPRC). Ann Oncol. 2018;29(8):1853-1860.