In the realm of lung cancer treatment, the mantra of precision medicine—the right drug for the right patient at the right time—offers a beacon of hope; promising to significantly increase patient survival rates. However, in practice, are we fully realizing its potential?

A study conducted by Sakamoto et al. serves as a crucial reference point for a discussion on biomarker testing for appropriate treatment selection in patients with advanced non-small cell lung cancer (NSCLC). This real-world study, conducted in a Japanese population, revealed that precision medicine may have reached a plateau.
This study documented a median overall survival (OS) of 24 months in patients with driver gene alterations who received targeted therapy, 15 months in patients with driver gene alterations who did not receive targeted therapy, and 11 months in patients with no driver gene alteration detected. However, it is important to note that this study confirmed biomarker status in only 86% (1,273/1,479) of patients. Of these, less than half—45%—of patients received single-gene testing, 33% received multigene testing, and 22% received both. Targeted therapy was administered as first-line treatment in 97% of EGFR-positive patients, 78% of ALK-positive patients, 63% of ROS-positive patients, 75% of BRAF-positive patients, and 63% of MET-positive patients, based on biomarker test results.1
We find similar results in other studies showing that biomarker testing for driver mutations is essential for improved treatment response and survival outcomes; however, it remains underutilized. To get past the plateau and achieve further improvements in survival, we must increase biomarker testing.

On one hand, the US Community-Based Oncology Practice Setting revealed that 62% of patients with a positive biomarker received targeted therapy. Additionally, 89% of patients had undergone at least one test, with 47% of patients undergoing both single-gene and next generation sequencing (NGS) testing, 34% of patients underwent single-gene testing, and 8% of patients underwent NGS testing. Notably, the study highlighted how testing rates increased when the use of a drug was approved in the country, with rates rising from 45% to 78% for ALK, 20% to 68% for BRAF, 35% to 56% for NTRK, and 30% to 74% for ROS1.2
On the other hand, the Prospective Registry of Biomarkers of Central Lung Cancer (LungPath) of the Spanish Society of Pathological Anatomy (SEAP), which analyzed 3,226 patients, observed, based on real-world evidence, that although the main biomarkers analyzed were EGFR (91%) and ALK (80%), ROS1 and PD-L1 were not determined in a significant portion of patients.3
This scenario can be replicated in the 16% of the world’s population in high-income countries, however, it is not a transferable reality in low- and middle-income countries, which account for most of the world’s population.4,5
Significant heterogeneity in molecular testing and targeted therapy implementation patterns was observed in these countries.6 Martin et al. described that 57% of patients with health insurance in Latin American countries underwent EGFR single gene testing. In comparison, only 41% of patients treated in the public sector had access to testing, but the rate varied between 28% and 79% across countries.7
If we want to have an objective analysis of the clonal evolution of the disease, is the mutagenic analysis of a single tissue block fixed in formalin and embedded in paraffin in metastatic patients sufficient? Or is it advisable to use a liquid biopsy to analyze tumour DNA drained from all sites of metastasis into the bloodstream? Liquid biopsy compensates for tissue insufficiency when it comes to molecular testing. It should be emphasized that pre-analytical processing and the selection of the appropriate technology according to the clinical context can condition the results obtained.8
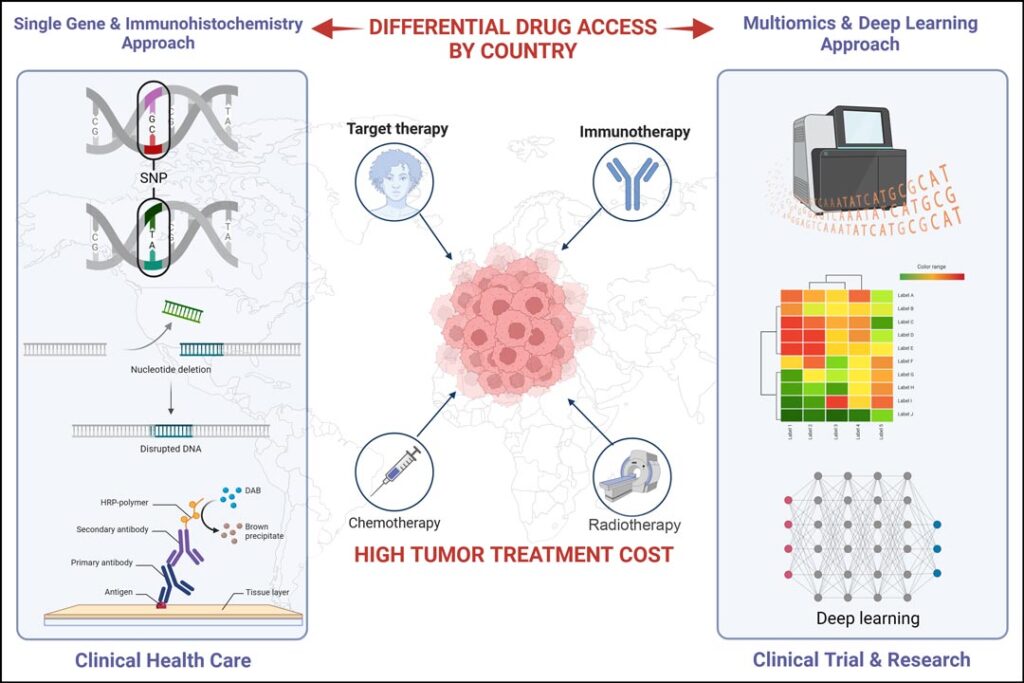
However, given the considerable amount of information obtained per patient from multi-omics cancer analysis and imaging, its holistic interpretation will require a deep learning approach associated with artificial intelligence.9 This approach is already being addressed in research, including clinical trials (see Figure 1).
The globalization of precision medicine programs in lung cancer poses significant challenges for healthcare systems. Molecular driver tests are associated with differential drug access by country.10 This is not just a financial issue but a matter of life and death for many patients. It is crucial to make these methodologies more accessible, as reducing inequality remains a core challenge for many countries.
Additionally, NGS reports contain large amounts of information that must be interpreted carefully before being used to make treatment recommendations. Practice guidelines such as those from the College of American Pathologists, the IASLC, the Association for Molecular Pathology, the American Society of Clinical Oncology, and the National Comprehensive Cancer Network help determine the most appropriate biomarkers and assays.
To conclude, studies analyzed in the real world demonstrate the potential of precision medicine to improve patient survival. However, precision medicine is currently at a plateau, and to move onward and upward, we must urgently reduce the costs of NGS and targeted treatments, address inequities, globalize access to approved drugs, and promote the use of deep learning to analyze the vast amount of available patient data. Progress is needed to select patients who would benefit from costly therapies with greater precision. This is not just a matter of scientific progress but a pressing need globally for the health and well-being of our patients.
References
- 1. Sakamoto T, Matsubara T, Takahama T, Yokoyama T, Nakamura A, Tokito T, Okamoto T, Akamatsu H, Oki M, Sato Y, Tobino K, Ikeda S, Mori M, Mimura C, Maeno K, Miura S, Harada T, Nishimura K, Hiraoka M, Kenmotsu H, Fujimoto J, Shimokawa M, Yamamoto N, Nakagawa K. Biomarker Testing in Patients With Unresectable Advanced or Recurrent Non-Small Cell Lung Cancer. JAMA Netw Open. 2023 Dec 1;6(12):e2347700. doi: 10.1001/jamanetworkopen.2023.47700.
- 2. Sireci AN, Krein PM, Hess LM, Khan T, Willey J, Ayars M, Deyoung K, Bhaskar S, Mumuney G, Coutinho A. Real-World Biomarker Testing Patterns in Patients With Metastatic Non-Squamous Non-Small Cell Lung Cancer (NSCLC) in a US Community-Based Oncology Practice Setting. Clin Lung Cancer. 2023 Jul;24(5):429-436. doi: 10.1016/j.cllc.2023.03.002.
- 3. Salas C, Martín-López J, Martínez-Pozo A, Hernández-Iglesias T, Carcedo D, Ruiz de Alda L, García JF, Rojo F. Real-world biomarker testing rate and positivity rate in NSCLC in Spain: Prospective Central Lung Cancer Biomarker Testing Registry (LungPath) from the Spanish Society of Pathology (SEAP). J Clin Pathol. 2022 Mar;75(3):193-200. doi: 10.1136/jclinpath-2020-207280.
- 4. https://data.worldbank.org/income-level
- 5. Werutsky G, Barrios CH, Cardona AF, Albergaria A, Valencia A, Ferreira CG, Rolfo C, de Azambuja E, Rabinovich GA, Sposetti G, Arrieta O, Dienstmann R, Rebelatto TF, Denninghoff V, Aran V, Cazap E. Perspectives on emerging technologies, personalized medicine, and clinical research for cancer control in Latin America and the Caribbean. Lancet Oncol. 2021 Nov;22(11):e488-e500. doi: 10.1016/S1470-2045(21)00523-4.
- 6. Hirsch FR, Zaric B, Rabea A, Thongprasert S, Lertprasertsuke N, Dalurzo ML, Varella-Garcia M. Biomarker Testing for Personalized Therapy in Lung Cancer in Low- and Middle-Income Countries. Am Soc Clin Oncol Educ Book. 2017;37:403-408. doi: 10.1200/EDBK_175243.
- 7. Martin C, Cuello M, Barajas O, Recondo G, Aruachan S, Perroud H, Sena S, Bonilla C, Orlandi F, Berutti S, Garcia Cocco V, Gomez A, Korbenfeld E, Zapata M, Cundom J, Orellana E, Goncalves S, Reinhold F. Real-world evaluation of molecular testing and treatment patterns for EGFR mutations in non-small cell lung cancer in Latin America. Mol Clin Oncol. 2022 Jan;16(1):6. doi: 10.3892/mco.2021.2439. Epub 2021 Nov 12.
- 8. Malapelle U, Pisapia P, Addeo A, Arrieta O, Bellosillo B, Cardona AF, Cristofanilli M, De Miguel-Perez D, Denninghoff V, Durán I, Jantus-Lewintre E, Nuzzo PV, O’Byrne K, Pauwels P, Pickering EM, Raez LE, Russo A, Serrano MJ, Gandara DR, Troncone G, Rolfo C. Liquid biopsy from research to clinical practice: focus on non-small cell lung cancer. Expert Rev Mol Diagn. 2021 Nov;21(11):1165-1178. doi: 10.1080/14737159.2021.
- 9. Rolfo C, Denninghoff V. Globalization of precision medicine programs in lung cancer: a health system challenge. .Lancet Reg Health Eur. 2023 Dec 12;36:100819. doi: 10.1016/j.lanepe.2023.100819.
- 10. Pennell NA, Arcila ME, Gandara DR, West H. Biomarker Testing for Patients with Advanced Non-Small Cell Lung Cancer: Real-World Issues and Tough Choices. Am Soc Clin Oncol Educ Book. 2019 Jan;39:531-542. doi: 10.1200/EDBK_237863.