Two large-scale, randomized controlled trials—the U.S. National Lung Screening Trial (NLST; n = 53,454) and the Dutch–Belgian Randomized Lung Cancer Screening Trial (NELSON; n = 15,792)—have demonstrated that lung cancer screening (LCS) with low-dose CT substantially reduces lung cancer mortality in a high-risk population.1,2 Despite the conclusive evidence for the effectiveness of LCS, national data indicate low uptake (≤4%) in the United States.3 UK programs using a more tailored approach (e.g., Lung Health Check) achieved higher uptake rates of 40% to 53%.4,5 Although not optimal, these rates are comparable to uptake rates for breast and colorectal cancer screening in the United Kingdom in low socioeconomic status (SES) groups, a population overrepresented among those at high risk for developing lung cancer.6,7 For the successful implementation of LCS, it remains an important challenge to ensure acceptance by the target population and adherence to initial as well as subsequent rounds of screening.
In this article, we describe factors contributing to low adherence rates in LCS programs and potential solutions from three different perspectives: detection of high-risk individuals, characteristics of eligible individuals, and screening-program features.
Risk-based Selection of Eligible Individuals
One initial barrier is the inability of primary healthcare systems and/or population-screening registries to consistently and systematically identify individuals on the basis of risk stratification (e.g., Prostate, Lung, Colorectal, and Ovarian [PLCO] risk—age, gender, educational level, smoking history, family history of lung cancer). Eligible individuals are more motivated to undergo screening when it is endorsed by a primary physician.8,9 However, many clinicians report (1) unfamiliarity with the eligibility criteria and the harms–benefits balance for several risk groups, (2) hesitance about the effectiveness of LCS, (3) negative emotional reactions of their patients, and (4) concerns about a potential increase in workload.10,11 Additionally, in many countries, the registration of smoking history in medical records is scarce or unreliable,12 which exacerbates the difficulty of reaching high-risk populations.
A recent study describes a successful increase in smoking-history coverage, from 66% to 86%, through LCS education offered to primary care groups and through a population dashboard in which clinic personnel are able to monitor smoking documentation.13 Important factors in this success have included improved data-management structures and recurring quality-improvement meetings. However, the smoking information had to be gathered retrospectively via phone calls or patient visits. To reduce the workload for primary care staff, such information could instead be captured through digitalized patient registration forms. Detection of eligible individuals could then be automated through reminders built into the electronic medical records system. Recent evidence suggests that primary care providers value an “all-in-one” platform that integrates smoking history, patient information relevant to shared decision-making, and a decision aid into the electronic health records system.14
Participant Factors
Individuals most susceptible for developing lung cancer (people with an extensive smoking history combined with lower SES) are less likely to access preventive care or cancer-screening programs.15 Individuals who currently smoke are more pessimistic about the survival chances for early-stage lung cancer and less likely to be reassured with a negative result.16,17 Internalized stigma and the fear of being judged may also deter people who smoke from screening.17 Often they are already worried about their heightened lung cancer risk,16 and messages in the education materials about their increased lung cancer risk might only provoke anxiety and prove counterproductive. They might benefit from positive messages—for instance, that early detection before symptom presentation can be effective in reducing lung cancer mortality. Furthermore, when smoking cessation is presented as an integral, mandatory part of the screening program, this could be off-putting to those who experience difficulties attempting to quit.17 Well-timed voluntary and personalized smoking-cessation services should be offered carefully.
In Western countries, long-time smoking is often associated with lower SES.18 Together with reduced health literacy and reduced comprehension of complex material, this can magnify barriers to LCS participation.19 A high information load can result in distrust, frustration, or fear. Low-burden, stepped, and personalized approaches are considered to be more effective in lower SES groups. In the Lung Screen Uptake Trial (LSUT), uptake increased for low-SES individuals when they received a leaflet with stigma-reducing and low-burden content followed by full information during the in-person visit, compared with those who received the standard information package.4 Uptake in regions of higher SES was, however, not positively influenced by the low-burden approach. Instead of using a one-size-fits-all approach, it is important to tailor the information materials according to the individual’s needs.20 In general, the more individualized the information is, the more likely the recipient will consider it personally relevant and engage with it.21
Additionally, individuals with lower SES are more likely to face barriers due to socioeconomic and geographical issues, such as travel time and costs, lack of insurance, and rigid and long work hours. Mobile CT scanners have the potential to make screening for underserved populations more accessible, if strategically located to reduce travel time. A recent feasibility study in the United States showed that mobile units were able to detect lung cancer at rates similar to those found in the NLST, with significant improvement of uptake in hard-to-reach groups, such as those identifying as African American, Hispanic, or Native American, or isolated rural groups.22
Multiple studies have shown that women are less likely to participate in LCS than men19,23 and to experience more cancer-related distress than men.24 Women may be more reluctant because of increased attention to women’s cancer-screening programs, such as cervical or breast cancer screening, and beliefs that lung cancer is a disease more relevant to men.24 However, a recent microsimulation study suggests that for LCS-eligible women, LCS is essential.25 Attendance of annual LCS was associated with 74% of the maximum benefit in life-years gained, which is almost five times higher than the benefit of colorectal cancer screening and nine times that of biennial mammography. The authors concluded that women with low compliance to screening guidelines were better off when screening for lung cancer even to the point of skipping other cancer screenings.
Program Factors
It remains a challenge to translate what has worked in the controlled environment of randomized trials to community-based screening programs. Although longitudinal adherence rates in the NLST and NELSON trials are high (90% to 95%), two recent pooled analyses of 12 international26 and 15 U.S.27 cohort studies demonstrated a mean adherence rate of only 28% and 55%, respectively.
The strongest independent predictor of longitudinal adherence in a recent U.S. cohort study was centralization.28 In comparison with decentralized programs, in which subjects are simply referred by a clinician to a low-dose CT examination, centralized programs use advanced data-management systems and employ program coordinators to support the screening process, track participants, and notify them when screening is due. In a retrospective analysis of an academic screening program, adherence increased from 22% to 66% after employing an LCS patient navigator.29 The patient navigator was responsible for shared decision-making conversations, smoking-cessation counseling, and tracking of participants. Similarly, adherence significantly increased in a randomized controlled study, in which the intervention group was supported by a patient-navigator trained in motivational interviewing, goal setting, problem solving, and smoking-cessation counseling.30
Conclusion: Moving Forward
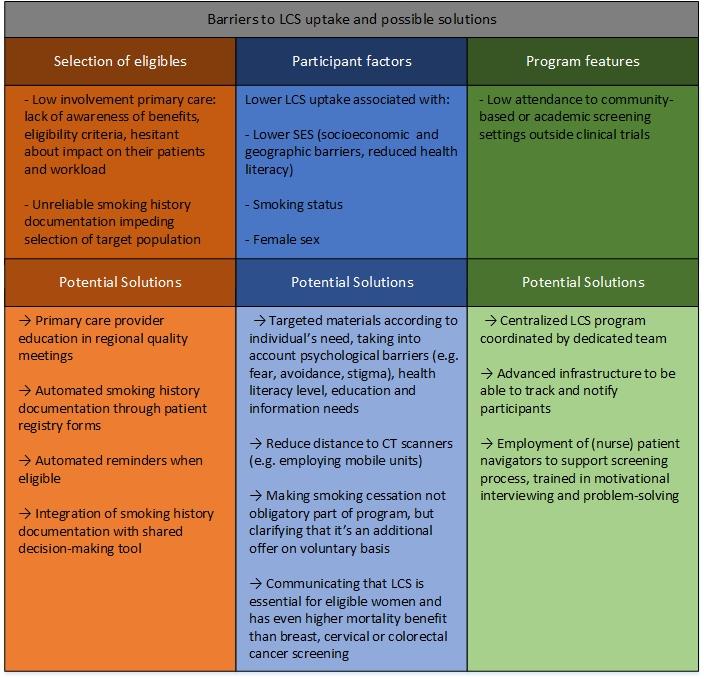
In Figure 1, factors associated with low LCS adherence and potential solutions are presented. A screening program can successfully recruit and retain participants if carried out by a committed team and supported by an advanced data-management system to track and notify participants. Education of primary healthcare staff to increase their involvement in the screening programs and early capture of smoking history data such as pack-years and cessation dates are essential to select and reach these hard-to-get groups.
Education materials should be targeted to the individual characteristics associated with low uptake, such as SES, sex, and smoking status. The personal approach of dedicated patient navigators is well-suited to address individual needs while more cost-effective, computer-tailored solutions are still being developed.31
Fig 1. Summary of Barriers to LCS Participation, and Potential Solutions.
- 1. National Lung Screening Trial Research Team, Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395-409.
- 2. de Koning HJ, van der Aalst CM, de Jong PA, et al. Reduced Lung-Cancer Mortality with Volume CT Screening in a Randomized Trial. N Engl J Med. 2020;382(6):503-513.
- 3. Tailor TD, Tong BC, Gao J, Henderson LM, Choudhury KR, Rubin GD. Utilization of Lung Cancer Screening in the Medicare Fee-for-Service Population. Chest. 2020;158(5):2200-2210.
- 4. a. b. Quaife SL, Ruparel M, Dickson JL, et al. Lung Screen Uptake Trial (LSUT): Randomized Controlled Clinical Trial Testing Targeted Invitation Materials. Am J Respir Crit Care Med. 2020;201(8):965-975.
- 5. Ghimire B, Maroni R, Vulkan D, et al. Evaluation of a health service adopting proactive approach to reduce high risk of lung cancer: The Liverpool Healthy Lung Programme. Lung Cancer. 2019;134:66-71.
- 6. Jack RH, Robson T, Davies EA. The varying influence of socioeconomic deprivation on breast cancer screening uptake in London. J Public Health (Oxf). 2016;38(2):330-334.
- 7. von Wagner C, Baio G, Raine R, et al. Inequalities in participation in an organized national colorectal cancer screening programme: results from the first 2.6 million invitations in England. Int J Epidemiol. 2011;40(3):712-718.
- 8. Schiffelbein JE, Carluzzo KL, Hasson RM, Alford-Teaster JA, Imset I, Onega T. Barriers, Facilitators, and Suggested Interventions for Lung Cancer Screening Among a Rural Screening-Eligible Population. J Prim Care Community Health. 2020;11.
- 9. Byrne MM, Lillie SE, Studts JL. Lung cancer screening in a community setting: Characteristics, motivations, and attitudes of individuals being screened. Health Psychol Open. 2019;6(1).
- 10. Margariti C, Kordowicz M, Selman G, et al. Healthcare professionals’ perspectives on lung cancer screening in the UK: A qualitative study. BJGP Open. 2020;4(3).
- 11. Leng J, Lei SF, Lei L, et al. Primary Care Providers’ Knowledge, Attitudes, Beliefs, and Practice Related to Lung Cancer Screening in Five High-Risk Communities in New York City. J Cancer Educ. 2020. [Epub ahead of print].
- 12. Polubriaginof F, Salmasian H, Albert DA, Vawdrey DK. Challenges with Collecting Smoking Status in Electronic Health Records. AMIA Annu Symp Proc. 2017;2017:1392-1400.
- 13. Peterson E, Harris K, Farjah F, Akinsoto N, Marcotte LM. Improving smoking history documentation in the electronic health record for lung cancer risk assessment and screening in primary care: A case study. Healthc (Amst). 2021;9(4):100578.
- 14. Reese TJ, Schlechter CR, Kramer H, et al. Implementing lung cancer screening in primary care: needs assessment and implementation strategy design. Transl Behav Med. 2021. [Epub ahead of print].
- 15. Kannan VD, Veazie PJ. Predictors of avoiding medical care and reasons for avoidance behavior. Med Care. 2014;52(4):336-345.
- 16. a. b. Quaife SL, Vrinten C, Ruparel M, et al. Smokers’ interest in a lung cancer screening programme: A national survey in England. BMC Cancer. 2018;18(1).
- 17. a. b. c. Quaife SL, Marlow LAV, McEwen A, Janes SM, Wardle J. Attitudes towards lung cancer screening in socioeconomically deprived and heavy smoking communities: informing screening communication. Health Expect. 2017 20(4):563-573.
- 18. Hiscock R, Bauld L, Amos A, Fidler JA, Munafò M. Socioeconomic status and smoking: a review. Ann N Y Acad Sci. 2012;1248:107-1`23.
- 19. a. b. Schütte S, Dietrich D, Montet X, Flahault A. Participation in lung cancer screening programs: Are there gender and social differences? A systematic review. Public Health Rev. 2018 39:23.
- 20. Noar SM, Benac CN, Harris MS. Does tailoring matter? Meta-analytic review of tailored print health behavior change interventions. Psychol Bull. 2007;133(4):673.
- 21. Kreuter MW, Holt CL. How do people process health information? Applications in an age of individualized communication. Current Directions in Psychol Sci. 2001;10(6):206-209.
- 22. Raghavan D, Wheeler M, Doege D, et al. Initial Results from Mobile Low-Dose Computerized Tomographic Lung Cancer Screening Unit: Improved Outcomes for Underserved Populations. Oncologist. 2020;25(5):e777-e781.
- 23. Raju S, Khawaja A, Han X, Wang X, Mazzone PJ. Lung Cancer Screening: Characteristics of Nonparticipants and Potential Screening Barriers. Clin Lung Cancer. 2020;21(5):e329-e336.
- 24. a. b. Brain K, Lifford KJ, Carter B, Burke O, McRonald F. Long-term psychosocial outcomes of low-dose CT screening: results of the UK Lung Cancer Screening randomised controlled trial. Thorax. 2016;71(11):996-1005.
- 25. Taksler GB, Peterse EFP, Willems I, et al. Modeling Strategies to Optimize Cancer Screening in USPSTF Guideline-Noncompliant Women. JAMA Oncol. 2021;7(6):885-894.
- 26. Lam ACL, Aggarwal R, Cheung S, et al. Predictors of participant nonadherence in lung cancer screening programs: a systematic review and meta-analysis. Lung Cancer. 2020;146:134-144.
- 27. Lopez-Olivo MA, Maki KG, Choi NJ, et al. Patient Adherence to Screening for Lung Cancer in the US: A Systematic Review and Meta-analysis. JAMA Netw Open. 2020;3(11):e2025102.
- 28. Sakoda LC, Rivera MP, Zhang J, et al. Patterns and Factors Associated With Adherence to Lung Cancer Screening in Diverse Practice Settings. JAMA Netw Open. 2021;4(4):e218559.
- 29. Spalluto LB, Lewis JA, LaBaze S, et al. Association of a Lung Screening Program Coordinator With Adherence to Annual CT Lung Screening at a Large Academic Institution. J Am Coll Radiol. 2020;17(2):208-215.
- 30. Percac-Lima S, Ashburner JM, Rigotti NA, et al. Patient navigation for lung cancer screening among current smokers in community health centers: a randomized controlled trial. Cancer Med. 2018;7(3) 894-902.
- 31. European Commission. 4-IN THE LUNG RUN: towards INdividually tailored INvitations, screening INtervals, and INtegrated co-morbidity reducing strategies in lung cancer screening. Updated September 17, 2021. Accessed September 28, 2021. https://cordis.europa.eu/project/id/848294