The KRYSTAL-12 trial confirmed earlier findings that adagrasib is beneficial in advanced KRAS G12C mutated non-small cell lung cancer (NSCLC). The phase III trial comparing adagrasib to docetaxel in patients with previously treated KRAS G12C-mutated NSCLC demonstrated statistically and clinically significant improvements in progression free survival (PFS), overall response rate (ORR), and intracranial efficacy compared with docetaxel. Results were presented at the 2024 American Society of Clinical Oncology Annual Meeting.

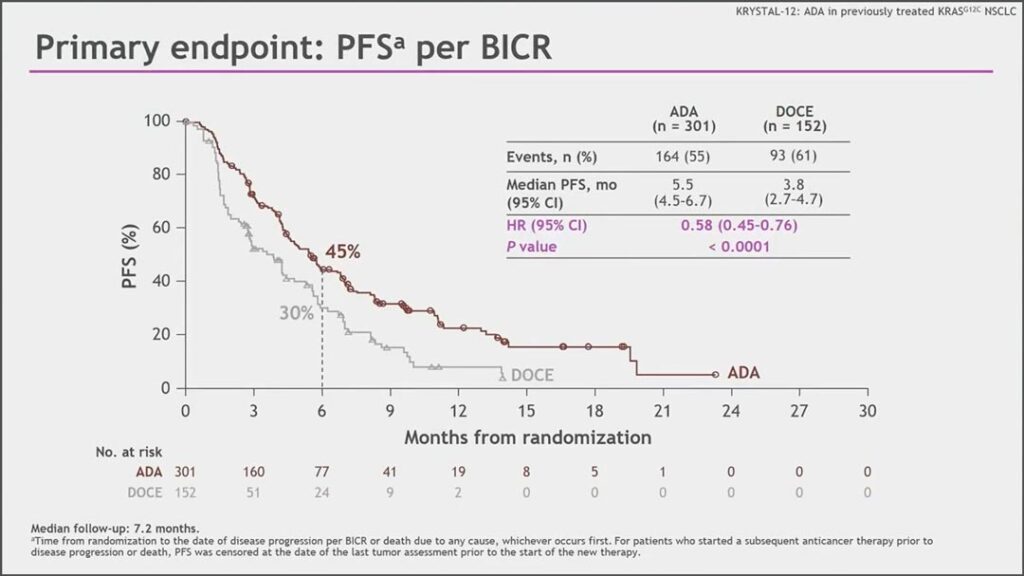
“We demonstrated superb results with adagrasib versus docetaxel; median progression free survival of 5.5 months versus 3.8 months respectively, with a hazard ratio of 0.58, and we observed this benefit across all subgroups,” said Tony Mok, MD, Chair and Li Shu Fan Professor of Clinical Oncology at the Chinese University of Hong Kong.
KRAS G12Cis among the most common driver oncogenes in lung cancer, seen in about 14% of all lung adenocarcinomas, Dr. Mok said. Adagrasib has already received accelerated approval in the US and conditional approval in the EU and UK for previously treated, advanced or metastatic KRAS G12C-mutated NSCLC based on phase II data. The initial findings from KRYSTAL-12 support those approvals, although overall survival (OS) data are not yet mature.
The open label KRYSTAL-12 trial randomized 453 patients with locally advanced or metastatic KRAS G12C-mutated NSCLC who had previously received chemotherapy and immunotherapy 2:1 to either adagrasib (301 patients) or docetaxel (152 patients). Stable brain metastases were allowed and patients in the docetaxel arm could switch to adagrasib upon confirmed disease progression. At data cutoff, 29% of docetaxel patients had switched to adagrasib, Dr. Mok said, and more crossover was expected as more patients experienced disease progression.
The primary endpoint was PFS. Secondary endpoints included ORR, Duration of Response (DoR), OS, safety, and patient-reported outcomes. The two arms were well balanced by age, sex, geographic region, and other characteristics. At baseline, 17% of patients had brain metastases, and 73% had received prior concurrent chemo-immunotherapy.
After a median of 7.2 months follow up, the median PFS by blinded independent review and investigator assessment were virtually identical with a hazard ratio of 0.58 (95% CI 0.45-0.76) and 0.57 (95% CI 0.45-0.74) favoring adagrasib, respectively
Dr. Mok pointed out that the Kaplan-Meier curves (see Fig. 1) separated early and remained separated throughout follow-up. The PFS benefits were similar across all subgroups; 32% of patients in the adagrasib arm responded to treatment compared to 9% in the docetaxel arm. Adagrasib yeilded a greater duration of response, he said, which translated into median DoR of 8.3 months versus 5.4 months.
Patients treated with adagrasib did experience more treatment-related adverse events (TRAEs) compared to patients in the docetaxel arm (94% vs. 86%). The most common adverse events were gastrointestinal, which has been previously associated with adagrasib. However, gastrointestinal toxicity was not severe. Dr. Mok said 53% of patients had diarrhea, but only 5% experienced grade 4 or higher in the adagrasib arm compared to 4% in the docetaxel arm. Additionally, TRAEs leading to discontinuation of treatment were seen in 7.7% patients in the adagrasib arm versus 14.3% in the docetaxel arm. He said toxicities were both consistent with prior reports on both agents and could be reversed with dose reduction or interruption.
“These results reinforce adagrasib as an efficacious treatment option for patients with KRAS G12C-mutated NSCLC after disease progression on prior chemotherapy and immunotherapy,” Dr. Mok said.