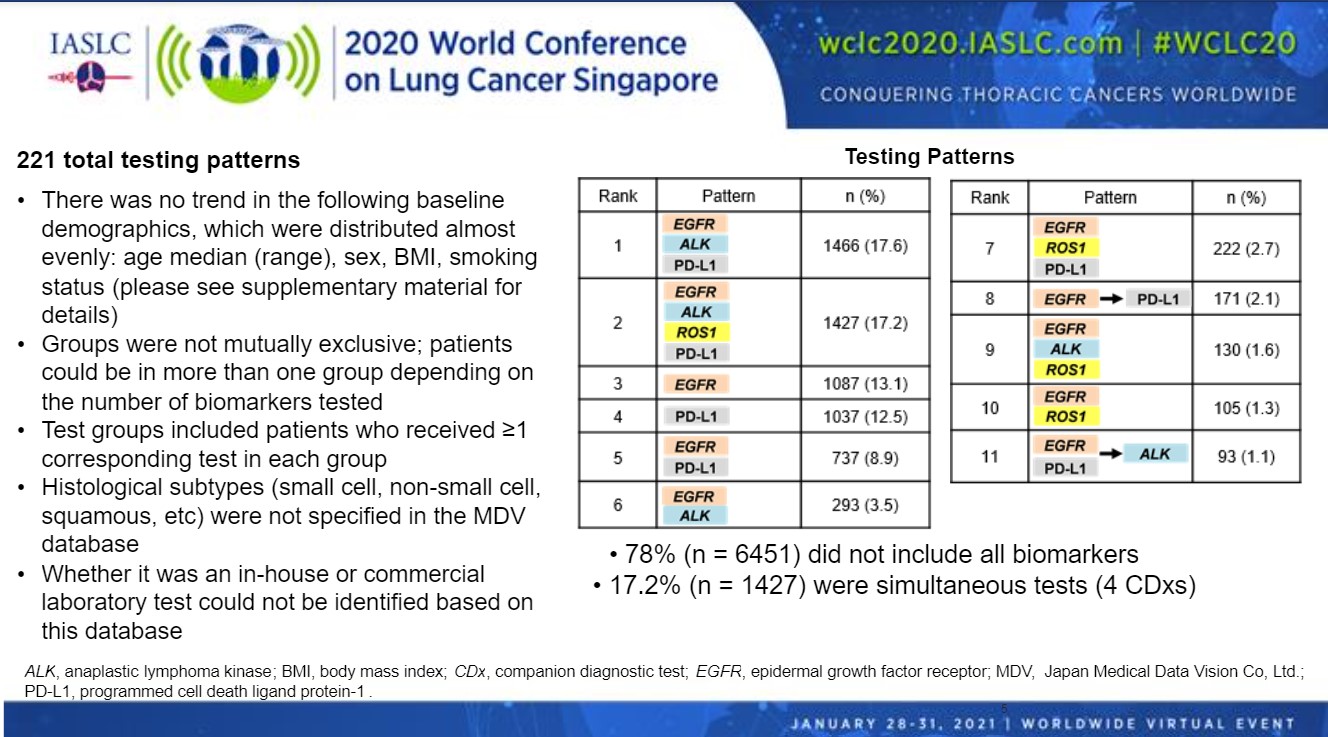
A significant number of patients with lung cancer did not undergo testing for all relevant biomarkers, according to real-world data from a study in Japan (MA08.08), and use of simultaneous testing compared with sequential testing may affect time to treatment.
Yasushi Yatabe, MD, PhD, of the department of diagnostic pathology, National Cancer Center Hospital, Tokyo, Japan, presented the results as part of the MA08: Advances in Biomarkers for Immune Checkpoint Blockade and Targeted Therapy in Non-Small Cell Lung Carcinoma.
Testing of EGFR, ALK, ROS1, BRAF, and PD-L1 biomarkers in patients with lung cancer is recommended in the CAP/IASLC/AMP, ASCO, and ESMO guidelines, with simultaneous testing encouraged to reduce the time between diagnostic testing and treatment. However, little real-world data are available on how testing, including implementation rates and biomarker patterns, is performed in clinical practice in Japan.
Dr. Yatabe and colleagues conducted this study to investigate the real-world patterns of single biomarker testing and subsequent treatment of patients diagnosed with lung cancer in Japan. The study was conducted before next generation sequencing was introduced.
The researchers looked at data from January 2014 to November 2018 for more than 13 million patients in the Japan Medical Data Vision Co, Ltd. Database, which covers 22% of major and middle-sized hospitals in Japan. They selected 560,451 patients with a lung cancer diagnosis. Of these, 23,747 had a test ordered and 12,877 had treatment ordered. The final study included 8,323 patients for analyzing molecular testing and patterns.
There were results for 221 total testing patterns, with the researchers identifying 11 common patterns (Figure). The most common testing pattern was for EGFR, ALK, and PD-L1 (17.6%) followed by testing for all four biomarkers EGFR, ALK, ROS1, and PD-L1 (17.2%). The proportion of simultaneous testing (17%) was lower than reported in the BRAVE study (32%), which was conducted at large specialized centers. This suggests that simultaneous testing is less common in the smaller hospitals included in our analysis, Dr. Yatabe said.
Testing for EGFR alone (13.1%), PD-L1 alone (12.5%) and EGFR and PD-L1 (8.9%) were also common. The remaining six testing patterns were combinations of these markers. Dr. Yatabe noted that these groups were not mutually exclusive. Patients could be in more than one group depending on the number of biomarkers tested.
“Use of sequential single-biomarker versus simultaneous testing may be an important barrier, requiring prioritization of individual biomarkers to test due to limited tissue sample availability,” Dr. Yatabe said.
There were no trends for baseline demographics for age, sex, BMI, or smoking status. Overall, 78% of patients did not undergo testing for all biomarkers.
The researchers also looked at how biomarker ordering patterns affected treatment. Time to treatment (TTT) was defined as period from the earliest testing order to first prescribed order date of anticancer drugs. The median TTT by number of tests suggested that a higher number of tests may be associated with a longer TTT, regardless of the prescribed therapy.
The overall TTT was 22 days. In patients that received a single test, the TTT was 21 days (range 2-509). This increased to 28 days (range 3-525) for those receiving two sequential tests, and 30 days (range 9-502) for those receiving three sequential tests.
The researchers noted a distinctive weekly patterns for biomarker testing regardless of prescribed drugs. These data suggested that physicians followed their work style on a weekly basis independent of the hospital size and geographical regions.
“Simultaneous testing of biomarkers in lung cancer and testing as early as possible may aid in the selection of appropriate and timely treatment, and ultimately improve the outcomes for patients,” Dr. Yatabe said.
Dr. Yatabe noted that some limitations of the study were that histological subtypes were not specified in the database nor was whether an in-house commercial laboratory test was used.
Changes over Time
Abstract discussant Lukas Bubendorf, MD, University Hospital Basel, Switzerland, said that the heterogenous biomarker testing patterns identified in this study of patients in Japan make the case for simultaneous compared with sequential testing.
“If you had several markers tested at the same time the time to treatment was lower than in cases of sequential testing where there were two or three rounds of testing,” Dr. Bubendorf said. “This was independent of whether targeted therapy or chemotherapy was used.”
However, the reasons for the variability seen remain obscure, he said. Similarly, the importance of the testing methods is not known. The presentation did not have information about whether immunohistochemistry, multiplex polymerase chain reaction (PCR), or next-generation sequencing were used.
“I assume that for mutational testing in Japan it is still mainly multiplex PCR,” Dr. Bubendorf said.
He also pointed out that the results of the study by Dr. Yatabe and colleagues also offers some opportunities.
“It will be interesting to see changes in pattern over time after the transition to next-generation sequencing, which is likely to happen at some point,”
Dr. Bubendorf said. “It is also important to clarify the role of IHC testing.”
This study was sponsored by Pfizer.