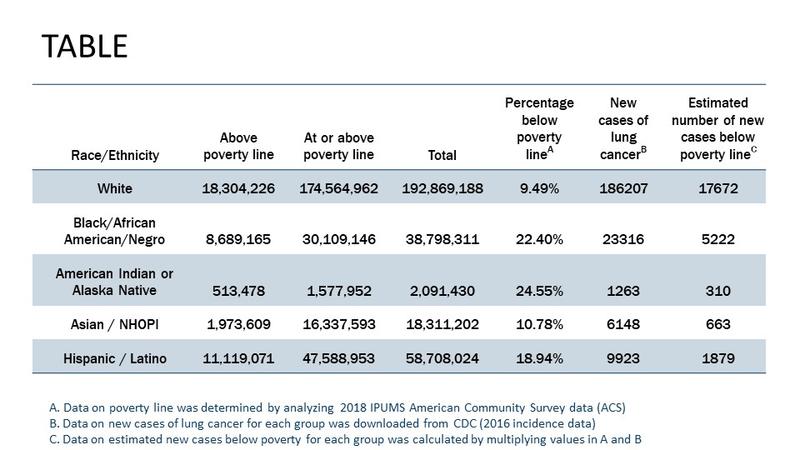
Lung cancer was first identified as a distinct disease in 1761.1 Since this discovery, we have come a long way—both in terms of progress towards understanding the heterogeneous biology of the disease and translating this understanding into action by development of new treatments.2 Indeed, since 2015, the United States Food and Drug Administration (FDA) has approved more than 25 new treatment approaches for lung cancer, including combination therapies and expanded approvals. With this progress has come the need to evaluate how lung cancer care is delivered and more importantly, to ensure that all patients have equitable access to adequate care to benefit from this progress. Defined as “a particular type of health difference that is closely linked with economic, social, or environmental disadvantage and affecting groups of people who have systematically experienced greater social or economic obstacles to health based on their racial or ethnic group, religion, socioeconomic status, gender, age, or mental health; cognitive, sensory, or physical disability; sexual orientation or gender identity; geographic location; or other characteristics historically linked to discrimination or exclusion,”3 health disparities in the lung cancer care continuum continue to persist and are well documented.4 When we look at recent lung cancer statistics in the United States (see Table), it is obvious that understanding health disparities requires us to look beyond a simplistic single construct focus on race and ethnicity. For example, the Table demonstrates that combining poverty data with race/ethnicity highlights the intersectionality of these two constructs to identify our most vulnerable patients with lung cancer.
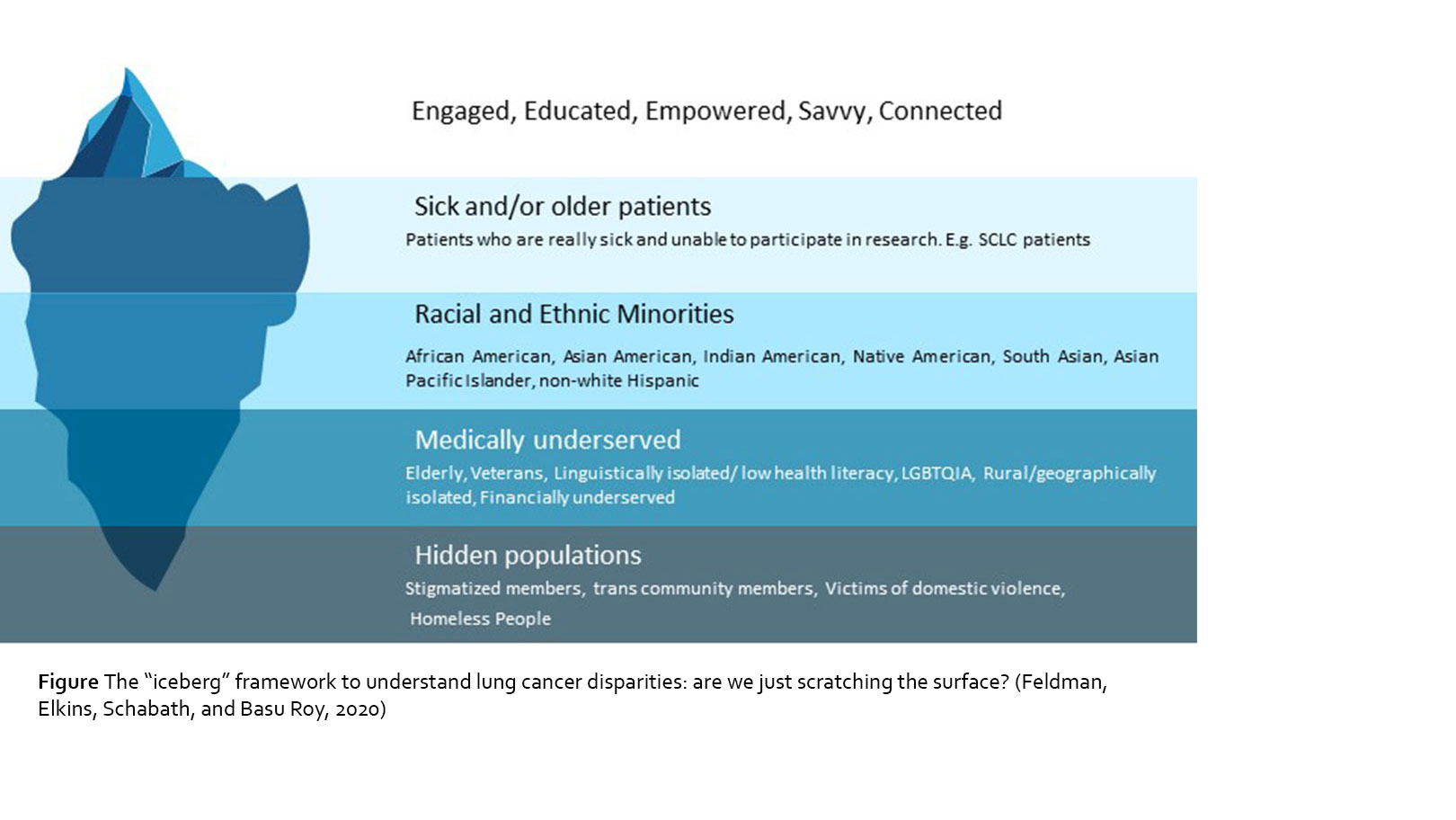
Despite the advancements in research and treatment for lung cancer, there are still vast disparities across the cancer care continuum, both in the United States and worldwide. There is a wide range of research documenting health disparities across numerous disparate, vulnerable, and underserved populations; however, understanding why these disparities persist, and more importantly, how they can be fixed to equalize and improve patient care in the real-world setting has not been well documented, especially given that these disparities are pronounced at several levels (see Figure – constructed based on the definition of health disparity provided in Healthy People 20203). As our communities become more diverse, it is increasingly important to recognize and address health disparities in all underserved communities.
Disparities in Lung Cancer Health Care Delivery
The increasing options and subsequent complexity of treating patients with lung cancer in the era of precision medicine have widened the health disparities gap even further, resulting in inconsistent administration of guideline-recommended screening or care. Health insurance coverage in the United States is one of the largest barriers to health care access and having health insurance is strongly correlated with better patient health outcomes. Compared to privately insured patients in the United States, those on Medicaid/other public insurance are significantly less likely to receive any systemic treatments (22% less likely), bevacizumab combinations (43% less likely), and tyrosine kinase inhibitors (30% less likely).5 Additionally, Black patients, compared with White patients, are more likely to present with more advanced disease where patients outcomes are much worse (as compared with patients diagnosed with early-stage disease). Black patients are also less likely to undergo surgery resection or receive radiation and systemic therapy, explaining a lower probability of 1-year survival.4
Another area of disparities that is often ignored is supportive care, including access to palliative care for pain management and psychosocial support. Prior studies suggest that communication with minority patients about prognosis, treatment options, and advanced care planning is suboptimal, which is a major barrier to the use of supportive care.6,7 The assessment and treatment of pain management is disproportionate among racial and ethnic minority groups, including access to pain medication and sometimes even the use of wrong medications for those in neighborhoods that are less likely to have adequate supply.7
Contemporaneously, the COVID-19 pandemic has exposed the already existing health inequities in underserved populations. It is well documented that Black patients, Indigenous People, patients of Pacific island descent, and Hispanic patients are 3.7 times, 3.5 times, 3.1 times, and 2.8 times, respectively, more like to succumb to COVID-19, than White patients.8 These disparities cannot be explained by differences in income alone.9 It is, therefore, very likely that the pandemic will only exacerbate lung cancer health care delivery gaps in these already disenfranchised communities.
The reasons for these disparities in lung cancer health care delivery are multifactorial and include systemic and institutional racism, inequitable access to health care, affordability, health care provider bias, patient trust, language and cultural barriers, and logistic barriers.10 Inequitable health care access is likely the single largest barrier to prevention, early detection, treatment information, and quality care in lung cancer. With respect to racial, ethnic, and cultural disparities, these constructs are often related to the quality of care due to socioeconomic status, lack of or inadequate access to health insurance coverage, language or cultural barriers, and provider implicit bias.11
Disparities in Lung Cancer Community Building
Support groups, both in-person12 and online13, have become a safe space for patients with cancer. Online communities that have been founded and maintained by patients with cancer allow a platform for candid discussions about access to care (such as how to seek second opinions and access to clinical trials), treatment experience (such as discussions on side effect management), and psychosocial peer-to-peer support. The lung cancer community itself has several such groups who identify themselves by the type of oncogene their cancer expresses. Despite the value added by these groups, disparities are also a major issue in both online and in-person support groups. Though these groups are international, previous studies looking at other patient advocacy groups suggest that the demographics of these communities are not representative of all patients.14 They tend to be comprised of patients with higher educational and wealth statuses, who are healthier, skew younger, and sometimes lack racial and ethnic distributions that are representative of the lung cancer patient community on a whole. In addition, technology challenges in accessing online communities exist for those in more rural areas, the elderly, and those who are socioeconomically disadvantaged.15 Patients belonging to racial and ethnic minorities and the LGBTQIA community might hesitate to join online communities which, they feel, may not recognize their distinct issues in health care.16 Sicker patients in all categories might not have the strength to participate in community building at all.17 Although the general feeling is that online communities are open to everyone, in reality they are not as inclusive as they might seem.
Disparities in Lung Cancer Clinical Research
It is important for us to have candid discussions about inclusiveness in lung cancer clinical research: is it a question of biology or equitable access to clinical trials or both? Progress in lung cancer has exploded during the past decade with the number of clinical trials, even in the first-line setting for newly diagnosed patients with lung cancer. Clinical trials are no longer considered a last-resort option. It is now well-documented that clinical trials may often be the best treatment option for patients with lung cancer (based on patient-specific and clinical factors). At the time of writing this article, the authors report that of the 884 interventional, recruiting clinical trials for treating non-small cell lung cancer, 488 of those trials were placed in United States, 251 in Western Europe, and only 9 in the African continent and 14 in South Asia, respectively.18 This suggests that placement of clinical trials globally in itself is an issue of access.
In the United States, lessons from other cancer types such as multiple myeloma suggest that patients from certain racial and ethnic groups may respond to cancer therapies differently than the majority of Caucasian/White populations,19 often the largest patient group in global oncology clinical trials.20 For lung cancer specifically, most clinical trials include too few Black and Hispanic patients.20 However, access to clinical trials is not equitable for various reasons. Lung cancer clinical trial locations are often in urban clinical settings and do not necessarily coincide with where most patients live and are treated. A study presented at The Science of Cancer Health Disparities in Racial/Ethnic Minorities and the Medically Underserved, led by the American Association for Cancer Research in 2019 reported that clinical trial placement for patients with both non–small cell lung cancer and small cell lung cancer in the United States do not overlap with where patients are located, based on zip code analysis of trial sites.21 This geographical isolation makes it difficult for patients to participate in trials,22 given the high cost associated with travel and lodging,23 and the high symptom burden of lung cancer. In addition, racial and ethnic demographics for most oncology clinical trials do not reflect the demographics of the disease incidence and mortality.
Finally, we cannot discount ageism in lung cancer clinical trials. The median age of diagnosis for lung cancer is 70. However, the average age of a lung cancer clinical trial participant is approximately 62 years, with this gap even wider in targeted therapy trials and in industry-sponsored phase III trials.24
Seeing Below the Surface
Given the disparities that exist, we therefore are only reaching a small fraction of patients with lung cancer as a community. We can use the iceberg graphic (see Figure) as analogy to depict the many layers of the patient community, including the visible and the more hidden. As you can see, engaged, educated and empowered patients are only the “tip of the iceberg.” These are the patients who are likely to have access to and receive best treatments, participate in online communities and join clinical trials. The majority of patients are below the “waterline” and deal with much difficulty in being seen and included, with a decreasing level of inclusivity that accompanies the patient populations that are submerged deeper in the ocean. Highest up towards the waterline are very sick patients. Although these patients might want to be engaged and connected, their illnesses prevent them from being as empowered as they might wish. It is difficult to advocate for yourself if you are too sick to have enough energy to take care of your own basic needs in life. Further down in the iceberg are racial and ethnic minorities, who deal with the frequent disparities we have mentioned. Even further down are the medically underserved patients, such as the elderly, veterans, the LGBTQIA population, and those who are isolated due to issues such as geography, finances, or low health literacy. The very bottom tip of the iceberg is submerged so far down that it is basically hidden; this includes populations that are stigmatized and often go unrecognized completely in health care such as members of the transgender community, homeless people, and victims of domestic violence.
It is, however, important to note that each layer of the iceberg does not in fact represent a singular construct of disparity. Rather, we need to use the lens of intersectionality25 to understand how each of the layers intersect and interact with each other to create an incredibly complex environment that creates and perpetuates health disparities. There is an immediate need for interventions that address the disparities, which are complex and reflect social and economic inequities more than just biologic differences.
Real-world Suggestions for Action

As patient research advocates, we must find a way to be more inclusive and create opportunities for health care delivery, community building and research to reach all of the populations who are currently “submerged” on the metaphorical iceberg. But how do we get there?
1. We must incorporate cultural humility into cancer care to foster equity and inclusion. Cultural humility is more than culture competence, which merely implies that providers understand cultural beliefs and values.26 Cultural humility is about critical self-reflection, accountability, and the commitment to learning from the patient. Lung cancer health care providers cannot make their own cultures the standard. They must take steps to understand each person in their own cultural context. We encourage all members of the health care team, including doctors, nurses, and social workers, to invoke the iceberg framework every time they meet and interact with a new patient.
2. We need multilevel interventions at local levels to address disparities and improve population health. A coordinated focus must include all stakeholders who provide interventions that affect health to enable high impact practices to occur at a local level. Only with such coordination can we have truly impact on interpersonal issues, organizational constructs, community building, education, occupational access, environmental health, and policy determination.
3. We must recognize and reduce disparities that exist in funding. Frequently, funding is granted to investigators and proposals from a relatively small group of recognized academic institutions.27 By proactively increasing the diversity of both institutions and scientists who apply for funding of lung cancer-specific proposals, we will ultimately affect disparities in patient care. More diversity in funding will naturally lead to an increase in novel topics of investigation, including those related to a more diverse group of patients.
4. We must develop data-driven initiatives to reduce and eliminate health disparities. As we discussed earlier, there is no paucity of data that documents health disparities. What is missing is how we can creatively include these data to develop intervention to reduce these disparities. It is important to note that such interventions will must be cooperative (health care providers and researchers, regulatory agencies, policy makers, and government officials), multilayered (individual-level, interpersonal, institutional, local, community-level, and national), and most importantly, include patient and community insights to be truly successful.
5. We should promote the use of novel technologies such as telemedicine either as part of accessing routine care or in the context of clinical trial participation. Telemedicine may help expand the reach of oncology care of geographically isolated or very sick patients. In addition, as part of clinical trial participation, telemedicine may be incorporated into steps such as remote informed consenting and patient monitoring, thereby minimizing patient travel to clinical trial sites and reducing the burden of clinical trial participation. Of course, it’s important to note that telemedicine may be helpful only in some circumstances. Also, we must ensure that telemedicine does not increase the digital divide.
6. Lastly, we must not forget the lung cancer itself is a health disparity that affects both a patient’s health-seeking behavior and a physician’s perception of a patient with lung cancer.28 This stigma must be addressed in order for us to make strides in equalizing other disparities associated with lung cancer. Infusing people from underserved communities throughout the health care system to include their perspective and input is critical to creating meaningful action that will impact systemic change, and to helping us better understand the three critical areas where disparities directly affect patient outcomes: health care delivery, community building, and research.
References:
- Witschi H. A short history of lung cancer. Toxicol Sci. 2001;64(1):4-6.
- Doroshow DB, Herbst RS. Treatment of Advanced Non–Small Cell Lung Cancer in 2018. JAMA oncology. 2018;4(4):569-570.
- Braveman P. What are Health Disparities and Health Equity? We Need to Be Clear. Public Health Rep. 2014;129 Suppl 2:5-8.
- Ryan BM. Lung Cancer Health Disparities. Carcinogenesis. 2018;39(6):741-751.
- Maguire FB, Morris CR, Parikh-Patel A, et al. Disparities in Systemic Treatment Use in Advanced-stage Non–Small Cell Lung Cancer by Source of Health Insurance. Cancer Epidemiol Biomarkers Prev. 2019;28(6):1059-1066.
- Johnson KS. Racial and Ethnic Disparities in Palliative Care. J Palliat Med. 2013;16(11):1329-1334.
- Meghani SH, Kang Y, Chittams J, et al. African Americans with Cancer Pain are More Likely to Receive an Analgesic with Toxic Metabolite Despite Clinical Risks: a Mediation Analysis Study. J Clin Oncol. 2014;32(25):2773-2779.
- APM. The Color of Coronavirus: COVID-19 Deaths By Race and Ethnicity in the US. (Updated August 5, 2020). www.apmresearchlab.org/covid/deaths-by-race?rq=COVID-19. Published 2020. Accessed August 8, 2020.
- Adhikari S, Pantaleo NP, Feldman JM, etal . Assessment of Community-Level Disparities in Coronavirus Disease 2019 (COVID-19) Infections and Deaths in Large US Metropolitan Areas. JAMA Netw Open. 2020;3(7):e2016938.
- Paskett E, Thompson B, Ammerman AS, et al. Multilevel Interventions To Address Health Disparities Show Promise In Improving Population Health. Health Aff (Millwood). 2016;35(8):1429-1434.
- Penner LA, Dovidio JF, Gonzalez R, et al. The Effects of Oncologist Implicit Racial Bias in Racially Discordant Oncology Interactions. J Clin Oncol. 2016;34(24):2874-2880.
- Gottlieb BH, Wachala ED. Cancer Support Groups: a Critical Review of Empirical Studies. Psychooncology. 2007;16(5):379-400.
- Gupta T, Schapira L. Online Communities as Sources of Peer Support for People Living With Cancer: A Commentary. J Oncol Pract. 2018:JOP.18.00261.
- Sadah SA, Shahbazi M, Wiley MT, et al. A Study of the Demographics of Web-Based Health-Related Social Media Users. J Med Internet Res. 2015;17(8):e194.
- Fareed N, Swoboda CM, Jonnalagadda P, et al. Persistent Digital Divide in Health-Related Internet Use Among Cancer Survivors: Findings from the Health Information National Trends Survey, 2003-2018. J Cancer Surviv. 2020;1-12.
- NLCN. Support Groups for Survivors. https://cancer-network.org/programs/support-groups-for-survivors/. Published 2020. Accessed October 11, 2020.
- Im EO. Online support of patients and survivors of cancer. Semin Oncol Nurs. 2011;27(3):229-236.
- CT.gov. Clinicaltrials.gov – sreach using terms Recruiting Studies | Interventional Studies | Non-small Cell Lung Cancer (October 11, 2020). Published 2020. Accessed October 11, 2020.
- Waxman AJ, Mink PJ, Devesa SS, et al. Racial Disparities in Incidence and Outcome in Multiple Myeloma: a Population-based Study. Blood. 2010;116(25):5501-5506.
- Loree JM, Anand S, Dasari A, et al. Disparity of Race Reporting and Representation in Clinical Trials Leading to Cancer Drug Approvals From 2008 to 2018. JAMA oncology. 2019;5 (10) e191870.
- 21. BasuRoy U, Regnante J, Xu L, et al. Geographic Relationship Between Lung Cancer Clinical Trial Sites and Patient Prevalence and Demographics in the Medicare Fee-for-Service Program. 2020; 29, (supp 2). Abstract D081.
- Feyman Y, Provenzano F, David FS. Disparities in Clinical Trial Access Across US Urban Areas. JAMA Netw Open. 2020;3(2):e200172.
- Unger JM, Gralow JR, Albain KS, et al. Patient Income Level and Cancer Clinical Trial Participation: A Prospective Survey Study. JAMA oncology. 2016;2(1):137-139.
- Ludmir EB, Mainwaring W, Lin TA, et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncology. 2019; 3;5(12).
- Lopez N, Gadsen VN. Health Inequities, Social Determinants, and Intersectionality. NAM Perspectives. https://nam.edu/health-inequities-social-determinants-and-intersectiona…. Published 2016. Accessed August 8, 2020.
- Greene-Moton E, Minkler M. Cultural Competence or Cultural Humility? Moving Beyond the Debate. Health Promot Pract. 2020;21(1):142-145.
- Hoppe TA, Litovitz A, Willis KA, et al. Topic Choice Contributes to the Lower Rate of NIH Awards to African-American/Black Scientists. Sci Adv. 2019;5(10):eaaw7238.
- Hamann HA, Ver Hoeve ES, Carter-Harris L, Studts JL, Ostroff JS. Multilevel Opportunities to Address Lung Cancer Stigma across the Cancer Control Continuum. J Thorac Oncol. 2018;13(8):1062-1075.