With no approved treatments for advanced NSCLC with EGFR exon 20 insertion mutations, treatment is mainly limited to chemotherapy, as these cancers don’t respond to traditional tyrosine kinase inhibitors (TKIs).
But previously treated patients with this rare subtype responded to mobocertinib, with a manageable safety profile, in a phase I/II trial discussed during (OA04: New Data from Rare EGFR Alterations).
The first-in-class, oral TKI is being explored post-chemotherapy for patients in this population with progressing disease, explained Caicun Zhou, MD, PhD, of Shanghai Pulmonary Hospital, an associate editor of IASLC Lung Cancer News and a current member of IASLC’s Board of Directors. Along with amavantamab and poziotinib, mobocertinib is one of three drugs in late-stage development to treat this population.1
In their open-label, multicenter investigation, researchers tested mobocertinib in patients with exon 20 insertion-mutated, locally advanced or metastatic NSCLC who were part of a dose escalation cohort (n=6), 7 expansion cohorts stratified by central nervous system involvement, treatment history and mutation location (n=22), and an extension cohort known as EXCLAIM (n=96).
All had an ECOG performance status of 0-1 and had received at least 1 prior line of treatment, which — with the exception of 10 EXCLAIM patients — included platinum-based chemotherapy.
Participants received 160 mg of mobocertinib once a day.
The primary endpoint was confirmed objective response rate (ORR) as assessed by an independent review committee (IRC). Secondary endpoints included safety, tolerability, and efficacy.
Study Findings
The 96 EXCLAIM patients were a median 59 years old (range: 27-80); 65% were female, 69% were Asian, and 49% had received 2 or more previous lines of therapy (range: 1–4). They spent a median 6.5 months on study treatment (range: 0–14).
Their IRC-confirmed ORR (all partial responses) was 23% (22; 95% CI: 15%–33%), while their investigator-assessed ORR was 32% (31; 95% CI: 23%–43%), Dr. Zhou said. Per IRC assessment, the group’s disease control rate (DCR) was 76% (73; 95% CI, 66%-84%). Fifty-three percent (51) had stable disease, 13% (12) had progressive disease and 12% (11) were not evaluable.
IRC-confirmed median progression-free survival (PFS) for the EXCLAIM patients was 7.3 months (95% CI, 5.5%-10.2%), Dr. Zhou said, and median time to response was 1.9 months (95% CI, 1.8-1.9), with the median duration of response (DOR) not yet reached.
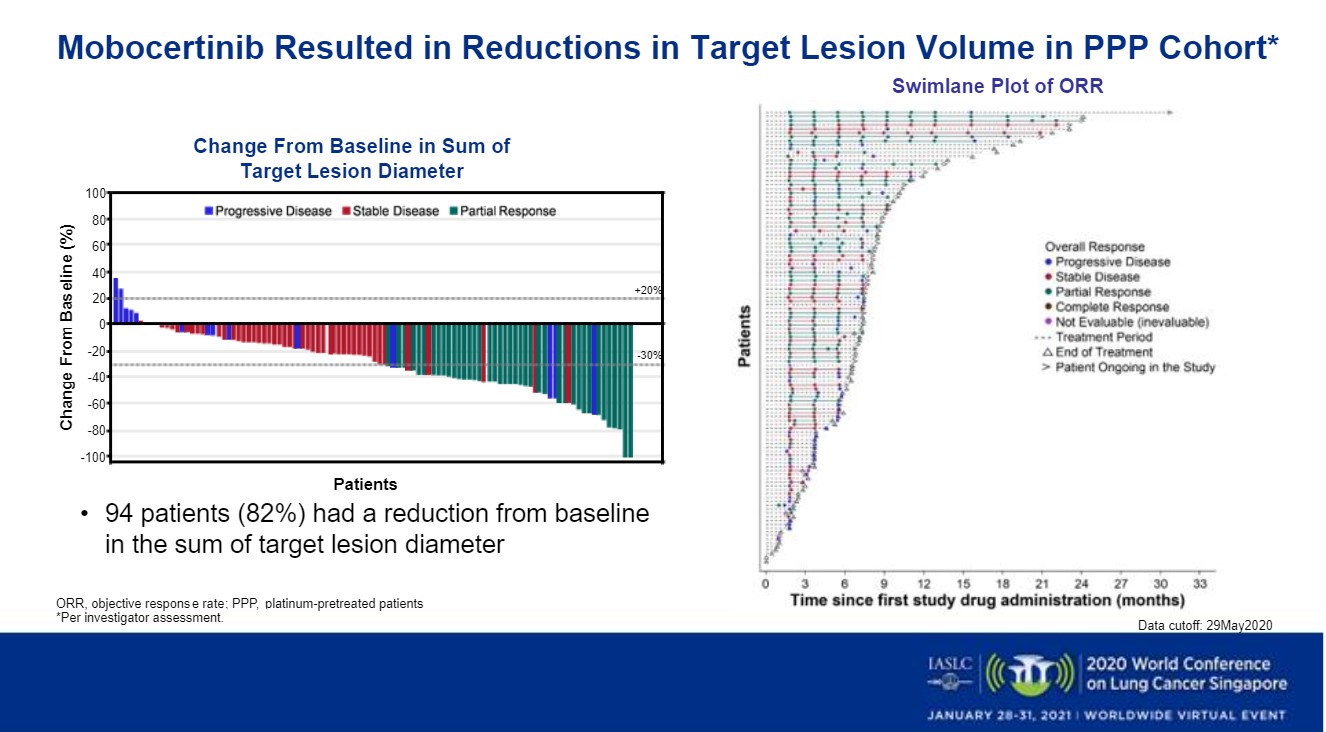
The researchers found similar outcomes for the platinum-pretreated patients (PPP) in the dose expansion, escalation and EXCLAIM cohorts (n=114), who were a median 60 years old (range: 27–84); 66% were female and 60% Asian. Fifty-nine percent had received 2 or more previous lines of systemic anticancer therapy (range: 1–7). Their median time on study treatment was 7 months (range: 0–31), with 38 (33%) continuing to receive the drug as of May 29, 2020.
The confirmed ORR for this group was 26% (30; 95% CI: 19%– 35%) and was 35% (40; 26%–45%) per investigator review, Dr. Zhou said. Their IRC-assessed DCR was 78% (89; 95% CI, 69%-85%). Twenty-six percent of the 114 (30) had a partial response, 52% (59) had stable disease, 11% (12) had progressive disease and 11% (13) were not evaluable.
The group’s confirmed median PFS was 7.3 months (95% CI, 5.5%-10.2%), with a 12-month PFS rate of 33% (95% CI: 21%-47%). Also per the IRC, median time to response was 1.9 months (95% CI, 1.8%-1.9%) and DOR was 17.5 months (8.3-NE).
Tumor shrinkage was observed in 82% of the PPP patients (Figure) and 80% of the EXCLAIM patients and was observed among all the study’s prespecified subgroups, Dr. Zhou said.
In the PPP versus EXCLAIM cohorts, 78% versus 84% of patients, respectively, had a confirmed DOR lasting beyond 6 months, and at data cutoff, over 50% of responses were ongoing across both groups, he said.
He added that additional follow-up with a data cutoff of Nov. 1, 2020 confirmed that ORR, DOR and PFS had held steady in both groups.
Adverse Events
Nearly all patients experienced treatment-related adverse events (TRAEs), most commonly diarrhea (90%), rash (45%), paronychia (34%), nausea (32%) and decreased appetite (32%). Grade 3 or higher TRAEs occurred in over 40% of patients, but only diarrhea affected 5% or more, Dr. Zhou said.
Nineteen PPP patients (17%) discontinued mobocertinib due to adverse events, as did 10 (10%) in the EXCLAIM group. One treatment-related death occurred in a platinum-pretreated patient in the EXCLAIM cohort.
A questionnaire showed that mobocertinib eased dyspnea, coughing and chest pain among EXCLAIM patients, he added.
Discussant Gillianne GY Lai, MBBS, of the National Cancer Centre, Singapore said that emerging traetments may fill unmet needs for patients with rare EGFR mutations. While Dr. Zhou and colleagues called mobocertinib’s safety profile manageable, Dr. Lai suggested that adverse events such as grade 3 diarrhea limit the drug’s utility.
She said that the ongoing phase 3 EXCLAIM study of mobocertinib versus platinum-based chemotherapy in treatment-naïve patients with exon 20-mutated NSCLC will shed more light on the drug’s usefulness.
Reference
- Hansen, T. Non-small cell lung cancer disease forecast and market analysis to 2035. Informa Pharma Intelligence. Published November 24, 2020. Accessed January 8, 2020. Non-small cell lung cancer (NSCLC) disease forecast and market analysis to 2035 | Report Store | Pharma intelligence (informa.com)