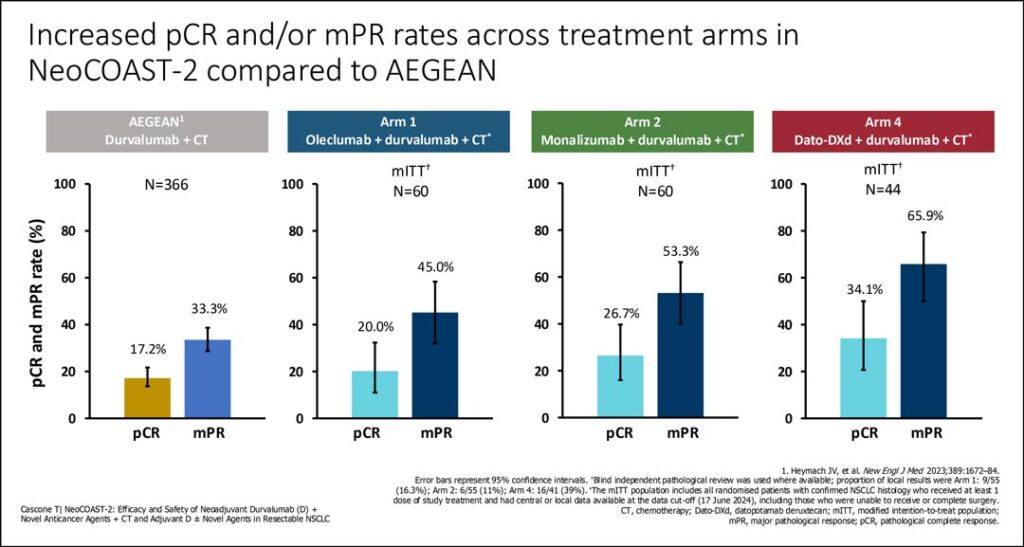
Early results from the open label NeoCOAST-2 trial demonstrated promising pathologic complete response (pCR) and/or major pathologic response (mPR) with novel neoadjuvant combinations including durvalumab/chemotherapy plus either oleclumab an anti-CD73 monoclonal antibody, or monalizumab, an anti-NKG2A agent, and durvalumab plus datopotamab deruxtecan (dato-DXd) plus single-agent platinum chemotherapy. This is the first report of neoadjuvant use of Dato-DXd, a TROP2-directed antibody-drug conjugate (ADC).

The novel perioperative ADC triplet combination had a stronger signal with a pCR rate of 34% and mPR rate of 66% compared to 20% and 45% for the oleclumab combination and 26% and 54% for the monalizumab combination, respectively. In the prior “historic control,” the AEGEAN phase III trial, durvalumab plus chemotherapy alone yielded a pCR rate of 17% and an mPR rate of 33%.
“This is the first global phase II study showing encouraging pathologic response rates for dato-DXd in the neoadjuvant setting for patients with resectable NSCLC,” said Tina Cascone, MD, PhD, Associate Professor Thoracic/Head and Neck Medical Oncology at the University of Texas MD Anderson Cancer Center, Houston. But she added that “Caution must be applied in using these results given the small sample size.”
NeoCOAST-2, an open label, multi-arm platform study, randomized 76 patients to neoadjuvant oleclumab, plus durvalumab plus chemotherapy, 72 patients to monalizumab plus durvalumab plus chemotherapy, and 54 patients to dato-DXd durvalumab plus chemotherapy followed by adjuvant therapy following lung cancer surgery.
A fourth arm randomized 70 patients to perioperative volrustomig, a PD-1/CTLA-4 bispecific antibody, plus chemotherapy. Data from the fourth arm will be reported at a later date.
All patients had resectable stage IIA – IIIB NSCLC with no evidence of EGFR mutations or ALK alterations. The primary endpoints were pCR and safety/tolerability. Key secondary endpoints included mPR and event-free survival (EFS) and the feasibility of surgery. The primary intent was to identify preliminary efficacy signals, Dr. Cascone noted, and the study was not powered for direct statistical comparisons between the arms.
The median age of patients across the three reported arms was 66 years old; about 60% were male. Most patients, about 65%, were white, but race was not reported for about 30% of study participants.
Roughly 70% of patients had PD-L1 expression ≥ 1%. More than 90% of patients underwent surgery; between 92% and 96% of surgeries yielded negative resection margins.
Response rates were higher in patients with PD-L1 expression ≥1% versus ≤1% across all three arms, Dr. Cascone reported, but she cautioned that sample sizes in all groups were small.
Response rates were higher in patients with PD-L1 expression ≥1% versus ≤1% across all three arms, Dr. Cascone reported. Safety profiles were, as expected, manageable in all three arms, she said. The most common adverse events included nausea, anemia, asthenia, and neutropenia.
“In perioperative NSCLC therapy these novel combinations demonstrated promising efficacy, with numerically higher pCR and/or mPR rates compared to historical benchmarks,” Dr. Cascone said. “All three study arms demonstrated manageable safety profiles and surgical rates comparable to currently approved regimens.”
Dr. Cascone presented the first results from NeoCOAST-2 during the first of two Presidential Symposia taking place during the 2024 World Conference on Lung Cancer.