The results of two studies presented at the 2021 World Conference on Lung Cancer—LIBRETTO-321 and ARROW—indicate that the selective RET inhibitors selpercatinib and pralsetinib are promising treatment options for patients in China with RET-fusion positive NSCLC.
According to the studies’ discussant Shengxiang (Henry) Ren, MD, PhD, of Shanghai Pulmonary Hospital, Tongji University, Shanghai, China, both studies were trying to answer the question of whether the positive data seen in global populations for novel RET inhibitor treatment could be generalized into the Chinese population.
“RET was identified as an oncogene three decades ago,” Dr. Ren said, with the first RET fusion mutation discovered in lung adenocarcinoma in 2012.
RET fusions are identified in about 1% to 2% of unselected patients with NSCLC and tend to occur in young patients and in those who have never chosen to smoke or who have only occasionally smoked. These fusions are detected mainly in lung adenocarcinoma and are associated with low tumor mutation burden and low response rate to immunotherapy.
Previously, multikinase inhibitors, such as cabozantinib and Lenvatinib, were evaluated in RET fusion-positive lung cancer, but had limited efficacy, with response rates of approximately 16% to 53% and a median duration of survival of 2.3 to 7.3 months.
The selective RET inhibitors selpercatinib and pralsetinib showed more potent activity in preclinical models and phase I/II studies.
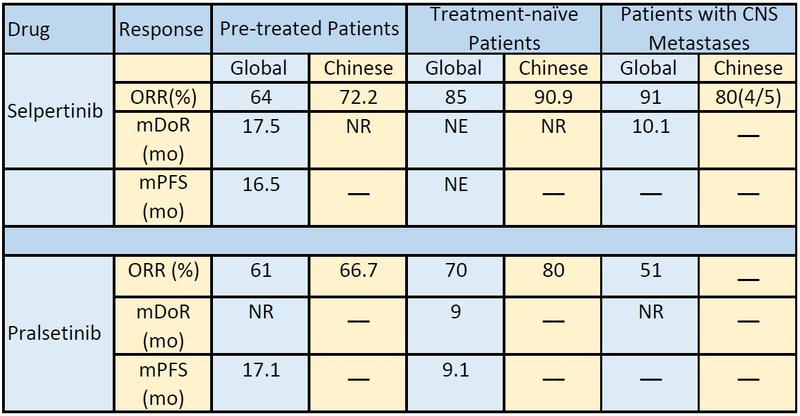
In a global population, the overall response rate (ORR) for selpercatinib was 64% in pretreated disease and 85% in treatment-naive disease. For pralsetinib, the ORR was 61% and 70%, respectively, Dr. Ren explained. Both selpertinib and pralsetinib had acceptable safety profiles. Now, the question remained if these results be generalized into Chinese populations?
LIBRETTO-321
Shun Lu, MD, of Shanghai Lung Cancer Center, Shanghai China, presented results from the phase-II LIBRETTO-321 trial of selpercatinib. In the study of 77 patients, selpercatinib demonstrated robust and durable antitumor activity, with an independent review committee (IRC)-assessed ORR of 69.2% in the primary efficacy analysis. The majority (94.4%) of responses were ongoing at a median follow-up of 9.7 months.
Patients aged 18 or older with treatment-naive or pretreated locally advanced or metastatic tumors were assigned to one of three patient cohorts. Cohort 1 included 30 patients with advanced RET-fusion NSCLC that had progressed on or was intolerant to one or more prior lines of standard first-line therapy or patients who declined or were unsuited for standard therapy. Cohort 2 included 26 patients with advanced RET-mutant medullary thyroid cancer with or without prior systemic therapy. Cohort 3 included 21 patients with advanced RET-altered solid tumors (18 patients with NSCLC).
Oral selpercatinib was administered at 160 mg twice daily in 28-day cycles. The primary efficacy analysis set included 26 patients with confirmed RET-fusion status. The analysis of all patients with NSCLC included 47 patients with RET fusion-positive who were assigned to either Cohort 1 or 3.
“The majority of patients in both pretreated and treatment-naive groups evidenced response to selpercatinib treatment,” Dr. Lu said.
Within the primary efficacy analysis set of patients the ORR was 61.1% in pretreated patients and 87.5% in patients with treatment-naive disease. Looking at all patients with NSCLC, the ORR was 66.0%; it was 58.3% for pretreated and 90.9% in treatment-naive disease.
Selpercatinib also showed intracranial response in patients with CNS lesions at enrollment. The confirmed intracranial response was 80%. Although Dr. Lu noted that interpretation of these results should be done with caution due to a limited number of patients (five patients).
“Selpercatinib showed durable response regardless of the treatment line,” Dr. Lu said.
The median treatment and tumor response was 1.8 months in the primary analysis and in all patients with NSCLC. Median duration of response was not reached in either group. More than 94% of patients in both arms remain in response.
Overall, selpercatinib was well tolerated with most adverse events being manageable and reversible among all 77 patients included in the trial. Only 3.9% of patients discontinued selpercatinib due to treatment-emergent adverse events considered to be related to the drug. About one-third (32.5%) of patients required a dose reduction. Only one patient had grade 5 treatment-emergent event (acute pancreatitis), which was considered not related to the drug.
“Selpercatinib is currently being evaluated as initial treatment in patients with advanced or metastatic RET-fusion positive NSCLC in the phase III LIBRETTO-431 trial,” Dr. Lu said.
ARROW
The second study looked at pralsetinib, a different drug targeting RET alterations. Pralsetinib was granted accelerated approval by the U.S. FDA in 2020 for adults with metastatic RET-fusion positive NSCLC and patients with advanced RET-fusion positive thyroid cancer.1
Qing Zhou, MD, PhD, of Guangdong Provincial People’s Hospital & Guangdong Academy of Medical Sciences, China, presented results from the ARROW study looking at the safety and efficacy in two of its eight cohorts: pretreated (30 patients) and treatment-naive (200 patients) Chinese patients with RET fusion-positive NSCLC.
“Pralsetinib demonstrated robust antitumor activities in RET-fusion positive [patients with] NSCLC regardless of prior therapies,” Dr. Zhou said.
The confirmed ORR was 66.7% in pretreated disease and 80.0% in treatment-naive disease. The clinical benefit rate was 84.8% and 86.7%, respectively.
Pralsetinib induced rapid and durable response in this patient population. Among the patients with prior platinum-based chemotherapy, the median treatment duration was 14.65 months. With a median follow-up of 17.0 months, 68.18% of those who demonstrated response remain on treatment. The median time to first response among those who demonstrated response was 1.89 months. The 6-month and 9-month duration of response were 77.3% and 50.0%, respectively.
In treatment-naive patients, the median treatment duration was 7.13 months. With a median follow-up of 8.2 months, 79.17% of those who demonstrated response remain on treatment. The median time to first response in this group was 1.87 months. The 6-month and 9-month durations of response were 76.7% and 38.3%, respectively. Overall, pralsetinib was well-tolerated in this group of Chinese patients with a manageable safety profile. All 68 patients had at least one treatment-emergent adverse events. Approximately 10% discontinued from treatment due to treatment-emergent adverse events.
“Pralsetinib is a promising targeted treatment with rapid and durable clinical activity in Chinese patients with RET fusion-positive NSCLC regardless of prior therapies,” Dr. Zhou said. “Pralsetinib should be a new standard of care for both Chinese patients and global patients with RET fusion-driven advanced NSCLC.”
Generalizability
“When comparing the global and Chinese populations we observed similar objective response rates in the pretreated and treatment-naive populations,” Dr. Ren said. “However, we still await follow-up on survival results and results of pralsetinib in Chinese patients with CNS metastases.”
Safety outcomes are also consistent between the global and Chinese populations, he added.
“In the era of immunotherapy, we know that chemotherapy plus immunotherapy is the standard of care for patients with advanced or metastatic NSCLC without EGFR/ALK mutations,” Dr. Ren said.
In addition to the LIBRETTO-431 phase III trial, the phase III AcceleRET Lung trial will also compare pralsetinib with a platinum chemotherapy-based regimen with or without pembrolizumab in RET-fusion positive advanced or metastatic NSCLC. The results of these studies will inform the use of these regimens in the first-line treatment of these patients.
- 1. U.S. Food and Drug Administration. FDA Approves Pralsetinib for Lung Cancer with RET Gene Fusions. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-appr…. Accessed October 13, 2021.






