Combination therapy with third-generation EGFR-TKI osimertinib plus savolitinib, a MET inhibitor, appears superior to osimertinib alone as first-line therapy for patients with MET aberrant, EGFR-mutated advanced non-small cell lung cancer (NSCLC). The phase II FLOWERS trial showed an objective response rate (ORR) of 90.5% and a disease control rate of 95.2% for combination therapy versus 60.9% and 87.0% for osimertinib monotherapy.

“Previous studies demonstrated that coexistence of EGFR mutation and MET amplification or overexpression reduces the sensitivity to EGFR-TKIs and is likely to be a key mechanism for mediating primary resistance to first-line EGFR-TKI monotherapy,” said Jin-Ji Yang, MD, Professor of Medical Oncology, Head of Division 1 of Pulmonary Oncology at the Guangdong Lung Cancer Institute at the Guangdong Provincial People’s Hospital, Southern Medical University, Guangzhou, China. “Osimertinib plus savolitinib demonstrated deeper and more durable responses throughout the study.”
Dr. Yang reported the findings from the phase II FLOWERS trial during his talk, Osimertinib with or without Savolitinib as 1L in de novo MET Aberrant, EGFRm Advanced NSCLC, at the 2024 World Conference on Lung Cancer. The presentation took place during the second of two Presidential Symposia on Monday morning.
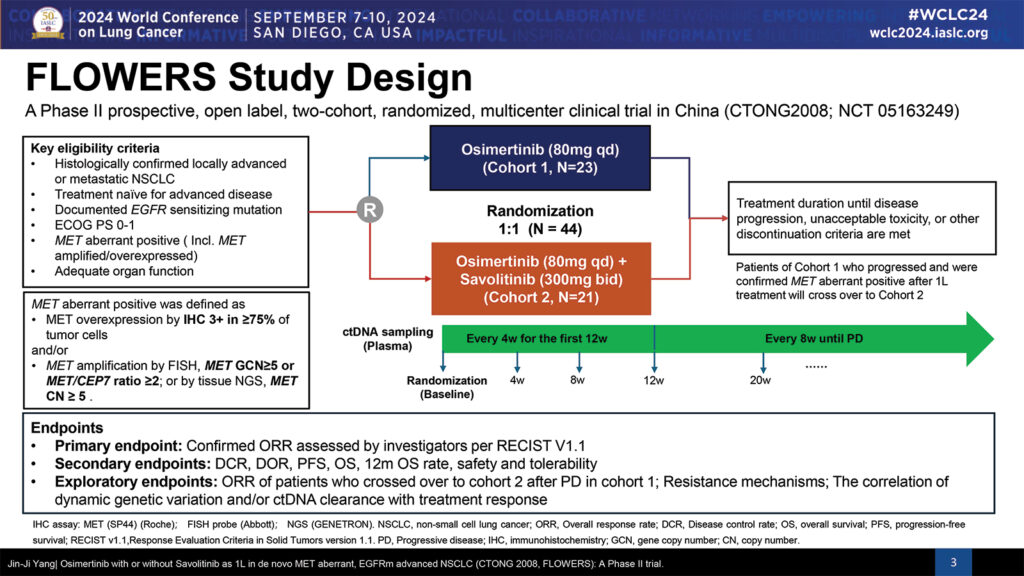
The open-label phase II trial included 44 patients, with roughly half receiving daily osimertinib alone and half (21 patients) being treated with daily osimertinib plus twice daily savolitinib. All patients had locally advanced or metastatic NSCLC, were treatment naïve for advanced disease, had a documented EGFR sensitizing mutation, and were MET aberrant, either MET amplified or MET overexpressed.
Treatment continued until disease progression, unacceptable toxicity, or other reasons for treatment withdrawal. Patients on monotherapy whose disease progressed and were confirmed MET aberrant had the option of crossing over to combination therapy.
The primary endpoint was confirmed ORR. Secondary endpoints included disease control rate (DCR), duration of response (DOR), overall survival (OS), 12-month OS rate, safety, and tolerability.
The median age of patients was 58 years. About half (48%) were female, and 66% had no smoking history. Most patients (96%) had stage IV disease; 34% had brain metastases at baseline. The most common EGFR mutations were 19 DEL (48%) and L858R (43%). Most tumors (82%) were MET overexpressed, 14% were MET amplified, and 5% were both MET overexpressed and amplified.
The median follow up at data cutoff was short, only 8 months, Dr. Yang said, and data are still immature. Preliminary data show trends favoring combination therapy, with 65% progression-free survival (PFS) at 12 months versus 49% for monotherapy. Preliminary PFS is 20 months for combination therapy versus 9 months for monotherapy, HR 0.59 (95% CI 0.91-1.81).
Adverse events were tolerable and manageable, Dr. Yang said. However, 100% of patients reported at least one treatment-related adverse event (TRAE), and TRAEs ≥ grade 3 were reported in 71% of patients in the combination arm versus 26% of patients in the monotherapy arm.
The most common TRAEs in the monotherapy arm were diarrhea, rash, and pruritus. Thrombocytopenia, peripheral edema, and ALT increase were most common in the combination therapy arm. There were no TRAEs leading to dose reduction or permanent discontinuation in the monotherapy arm compared to 24% in the combination therapy arm. There were no TRAE-associated deaths.
“This combination regimen has potential to provide a novel first-line treatment option for such patients,” Dr. Yang said.