Prolonging low-dose CT intervals for individuals with heavy smoking histories, based on the results of their baseline scans, is a safe and effective way to reduce unnecessary screening and treatment, a research team has found.
Ugo Pastorino, MD, and colleagues have demonstrated the safety of 2- and 3-year screening intervals for individuals with negative baseline screens and explored adding strategies to CT screening – namely, the evaluation of microRNA signatures and the application of cancer-prevention measures.
In his May 7 presentation “Advance Lung Cancer Screening Globally,” Dr. Pastorino, co-chair of the CT Screening Symposium and director of the Thoracic Surgery Division at the Istituto Nazionale Tumori in Milan, discussed the MILD, BioMILD, and SMILE trials. He also is chair of the IASLC’s Screening and Early Detection Committee.
MILD
The Multicentric Italian Lung Detection trial randomly assigned 2,376 participants to annual or biennial screening and found no disadvantage to the 2-year interval for those with negative findings at baseline.
Those participants were included in the biennial screening arm, while individuals with questionable or positive initial screening results received annual low-dose CT.
The two arms demonstrated similar overall (HR 0.80, 95% CI [0.57-1.12]) and lung cancer-specific (HR 1.10, 95% CI [0.59-2.05]) mortality at 10 years. However, compared with annual screening, biennial scans avoided 44% of follow-up CTs in negative-baseline participants, as well as 38% of CTs across all study participants, without any increase in the occurrence of stage II to IV or interval lung cancers, the researchers found.
“The MILD trial provides original evidence that prolonged screening beyond 5 years with biennial low-dose CT can achieve a lung cancer mortality reduction comparable to annual low-dose CT in subjects with a negative baseline examination,” the researchers wrote in a 2019 paper published in the European Journal of Cancer.1
BioMILD
The BioMILD Trial also tested an extended screening interval for those with negative baseline scans, evaluating the combined utility of low-dose CT and blood microRNA signatures at baseline to predict lung cancer risk.
Participants were 50 to 75 years old with a heavy past-decade smoking history,2 Dr. Pastorino said. Those whose CT and miRNA were negative at baseline were assigned to screening every 3 years to determine whether that would reduce stage 1 diagnoses or increase the number of unresectable cases or interval cancers identified.
Based on 24 miRNA signatures, participants were divided into three levels of risk. A low score was considered a negative screen and an intermediate or high score positive. In evaluating CT findings, lesions larger than 113 mm3 were considered indeterminate and those larger than 260 mm3 positive.
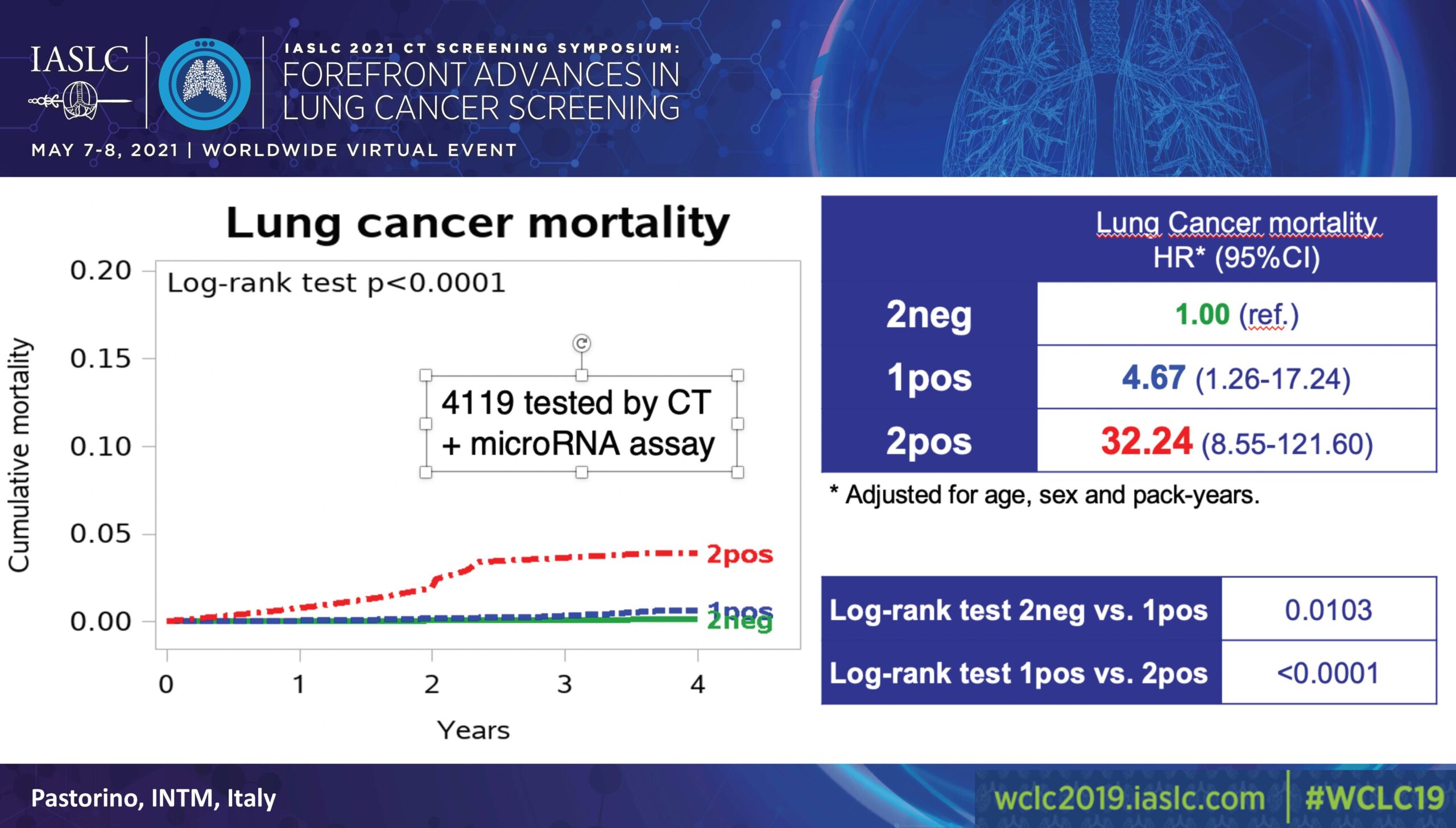
Of 4,119 participants, 58% (2384) had both negative CT and miRNA results; 37% (1526) had either a positive CT or miRNA; and 5% (209) had both a positive CT and miRNA (Figure).
A 2019 interim analysis, which used double-negative cases as a reference valued at 1.00, found that participants with a positive CT or miRNA faced a risk of 5.96 (3.38-10.52) and those with both faced a risk of 36.64 (20.31-66.11; p˃0.0001).
In evaluating lung cancer mortality, also using double-negative cases as a reference valued at 1.00, the analysis found that participants with a positive CT or miRNA faced a risk of 4.67 (1.26-17.24) while those with both faced a risk of 32.24 (8.55-121.60; p˃0.0001).
The researchers implemented active surveillance of subsolid lesions in 225 individuals. “Among these, 3% developed lung cancer, and there were no deaths. So, active surveillance was confirmed as a valid option for subsolid lesions,” Dr. Pastorino said.
An April 2021 update of the trial’s results confirmed the safety of the 3-year screening interval for the target population. During a median follow-up of 5.3 years, 85 cancers were found, with no increase in advanced-stage cases, unresectable disease, or interval lung cancer, Dr. Pastorino said.
SMILE
The Screening and Multiple Intervention on Lung Epidemics trial, launched in July 2019, will assess the effectiveness of low-dose CT scans combined with disease-prevention interventions.
Individuals aged 55 to 75 with heavy smoking histories are being randomly assigned to one of several interventions: aspirin plus diet modification and physical activity to reduce inflammation; the smoking-cessation drug cytisine; or all those tactics. Every participant will undergo low-dose CT, including a control group that will receive no additional intervention.3 Endpoints include smoking cessation and C-reactive protein levels in relevant trial arms at 12 and 24 months, respectively.
Dr. Pastorino suggested that future research build on the understanding that it’s safe to institute targeted screening intervals after baseline and that “combining prevention with low-dose CT screening is a feasible option to reduce mortality for all causes, which we know is very important in heavy smokers of that age.”
- 1. Pastorino U, Sverzellati N, Sestini S, et al. Ten-year results of the Multicentric Italian Lung Detection trial demonstrate the safety and efficacy of biennial lung cancer screening. Eur J Cancer. 2019;118:142-148.






