Approximately 10% of “real-world” patients with malignant pleural mesothelioma (MPM) benefitted from checkpoint inhibitor therapy with nivolumab, according to the results of a trial presented by Robert A. Belderbos, MD, of Erasmus Medical Center, the Netherlands, during OA09: Mesothelioma from Pathogenesis to Therapy.
MPM is an uncommon cancer with low survival rates between 9 to 12 months with first-line chemotherapy treatment. In lung cancer, immunotherapy has led to significant increases in survival. In MPM, however, checkpoint inhibition has shown clinical activity in phase II trials but a randomized study (Promise-MESO) was negative.
Dr. Belderbos and colleagues selected real-world patients that had early access to nivolumab for second or third-line treatment of MPM. Treatment was given independent of PD-L1 expression.
Median progression-free survival (PFS) for the entire cohort was 2.3 months, with a 6-month PFS rate of 23%. The median overall survival rate (OS) was 6.7 months, with a 1-year OS of 31%.
To put this in context, an abstract for the phase III CONFIRM study presented at the Presidential Plenary, showed that nivolumab significantly improved both PFS and OS compared with placebo in patients with relapsed malignant mesothelioma. The median PFS with nivolumab in CONFIRM was 3.0 months and the median OS was 9.2 months.
Dr. Belderbos and colleagues also looked at survival by histological subtype: epithelioid vs. non-epithelioid. The PFS was similar between the two groups. There was a trend towards worse OS in patients with non-epithelioid disease, but it was not statistically significant. The median OS was 7.4 months for epithelioid tumors compared to 4.8 months for non-epithelioid tumors (p = 0.082).
Based on historical data, it is known that patients with non-epithelioid histology have worse OS if left untreated or treated with first-line chemotherapy, Dr. Belderbos said.
“In our data we do not see this difference,” he said.
Recent data from the Checkmate 743 study showed that combination immunotherapy seemed to be more effective in non-epithelioid tumors than in epithelioid tumors.
“Maybe the lack of significant difference in our data between the epithelioid and non-epithelioid tumors, whilst this is expected based on historical data, might hint toward more effectiveness in non-epithelioid tumors for anti-PD-L1 treatment,” Dr. Belderbos said.
From 33 out of 107 patients PD-L1 expression was available. A positive PD-L1 expression was defined as 1% expression or more. Looking at outcomes by PD-L1 expression there was a trend toward better and increased OS for patients with PD-L1 expression. The median PFS was 4.2 months for PD-L1 positive tumors compared to 1.7 months for PD-L1 negative tumors.
When they looked at PD-L1 expression according to best overall response they found that there was a relative over representation of patients with PD-L1 positive tumors in the partial responder group. Overall response rate was 36% for patients with a PD-L1 positive tumor and 9% for patients with a PD-L1 negative tumor.
Next, to better elucidate the importance of radiologic response to nivolumab, the researchers divided patients into three groups according to their radiologic response and compared PFS and OS between these groups. For PFS, only patients with partial response and stable disease were included and, for OS, the researchers only selected patients that were still alive 12 weeks after start of treatment to avoid immortal time bias.
In the partial response group (10 patients), with a median follow-up of 14.1 months, there were no deaths reported and only two patients had progressive disease. Median OS was not reached for patients with partial response. For those with stable disease, median OS was 10.2 months and for progressive disease it was 6.2 months.
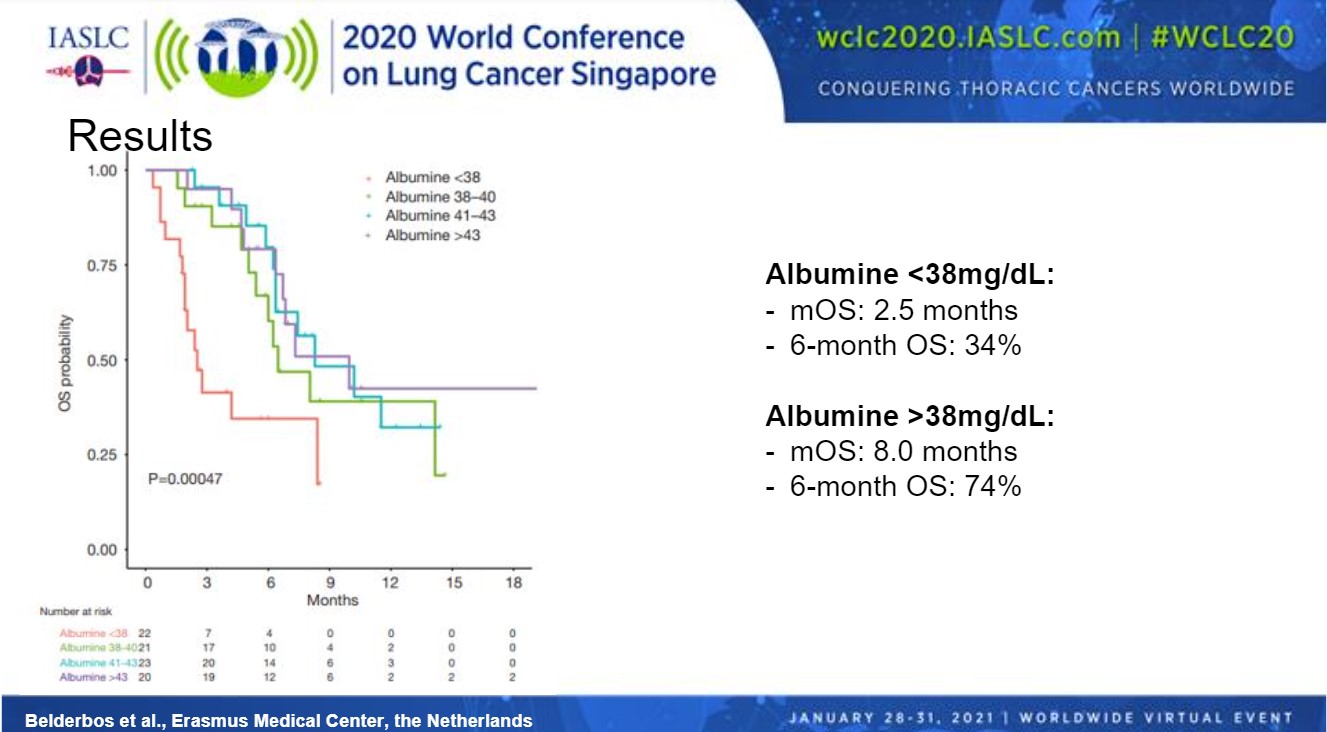
After multivariable analysis with clinical parameters and peripheral blood parameters collected at baseline, albumin was the only significant prognostic factor for OS. Therefore, the researchers divided patients into quartiles according to their albumin values (Figure). Patients in the lowest quartile (< 38 mg/dL) had a shorter median OS compared with patients in the other groups. Median OS was 2.5 months for patients with albumin levels < 38 mg/dL compared with 8.0 months for patients with albumin levels > 38 mg/dL. Six-month OS was 34% in the lower albumin group compared with 74% in the higher group.
To conclude, Dr. Belderbos said that patients with a radiologic response do seem to have clinical benefit from nivolumab in terms of improved PFS and OS.
He added, “PD-L1 expression and low albumin levels at baseline are negatively related to survival and might help us identify patients with low chance of having clinical benefit from nivolumab treatment. Future studies should include immune-monitoring of peripheral blood as well as tumor material to fully understand the immunological mechanism that predicts response and that could lead to new combination treatments in the future and might help us identify patients that do respond to immunotherapy.”
The study discussant, Thomas John, MBBS, PhD, Peter MacCallum Cancer Centre, Melbourne Australia, said that among the take-aways of this study are the results looking at histology.
“If you have non-epithelioid histology immunotherapy seems to push your survival closer to epithelioid histology, which in itself is an achievement,” Dr. John said.
In general, single-agent PD-L1 inhibition has shown similar results across several studies, he said. Across the board, response rates and survival are not particularly high. That begs the question: should immunotherapy be used in mesothelioma?
“Results from Checkmate 743 show that there is a place for immunotherapy but demonstrated that, perhaps, dual-agent treatment is better,” Dr. John said.
This session had a real-time Q&A that provided attendees with the opportunity to ask questions of the session participants. The Q&As are included in the On-Demand recordings, available through the virtual platform. Registration is ongoing for the next 60 days at wclc2020.iaslc.org.