By now, investigators, sponsors, clinicians, and patients are widely aware of the crisis facing cancer clinical trials. With scientific discoveries occurring more rapidly than ever, there is a growing need to bring novel therapies to patients as efficiently as possible. Yet, in the United States, fewer than 3% of adults with cancer enroll in clinical trials, leading to delayed or incomplete study results, rising drug development costs, nongeneralizable findings, and a growing public frustration with the process.1-4
Several factors contribute to the persistent and increasing inefficiencies. Cancer clinical trials are becoming increasingly complicated, with more procedures required in ever-narrowing time intervals.5 As trials become more complex, they may be less available both to individual patients (due to the cost and time of participation) and to entire institutions (due to feasibility).6 These issues disproportionately affect underrepresented minority populations, who face the greatest burden of lung cancer morbidity and mortality.7,8
If there is a single dominant issue hindering cancer clinical trials, it is surely the use of increasingly stringent and often poorly justified eligibility criteria. Indeed, it is far more likely that a patient does not qualify for a trial than chooses not to participate.9 Despite calls for simplification, the number of enrollment criteria in U.S. National Cancer Institute–sponsored lung cancer clinical trials continues to rise.5 These trends have particular impact on patients with lung cancer, who tend to be elderly, have extensive smoking history, and have multiple comorbidities, leading not only to low accrual rates but also to limited generalizability of trial results. To address these concerns, organizations such as LUNGevity, Friends of Cancer Research, and the American Society of Clinical Oncology have critically evaluated trial design and implementation.10,11
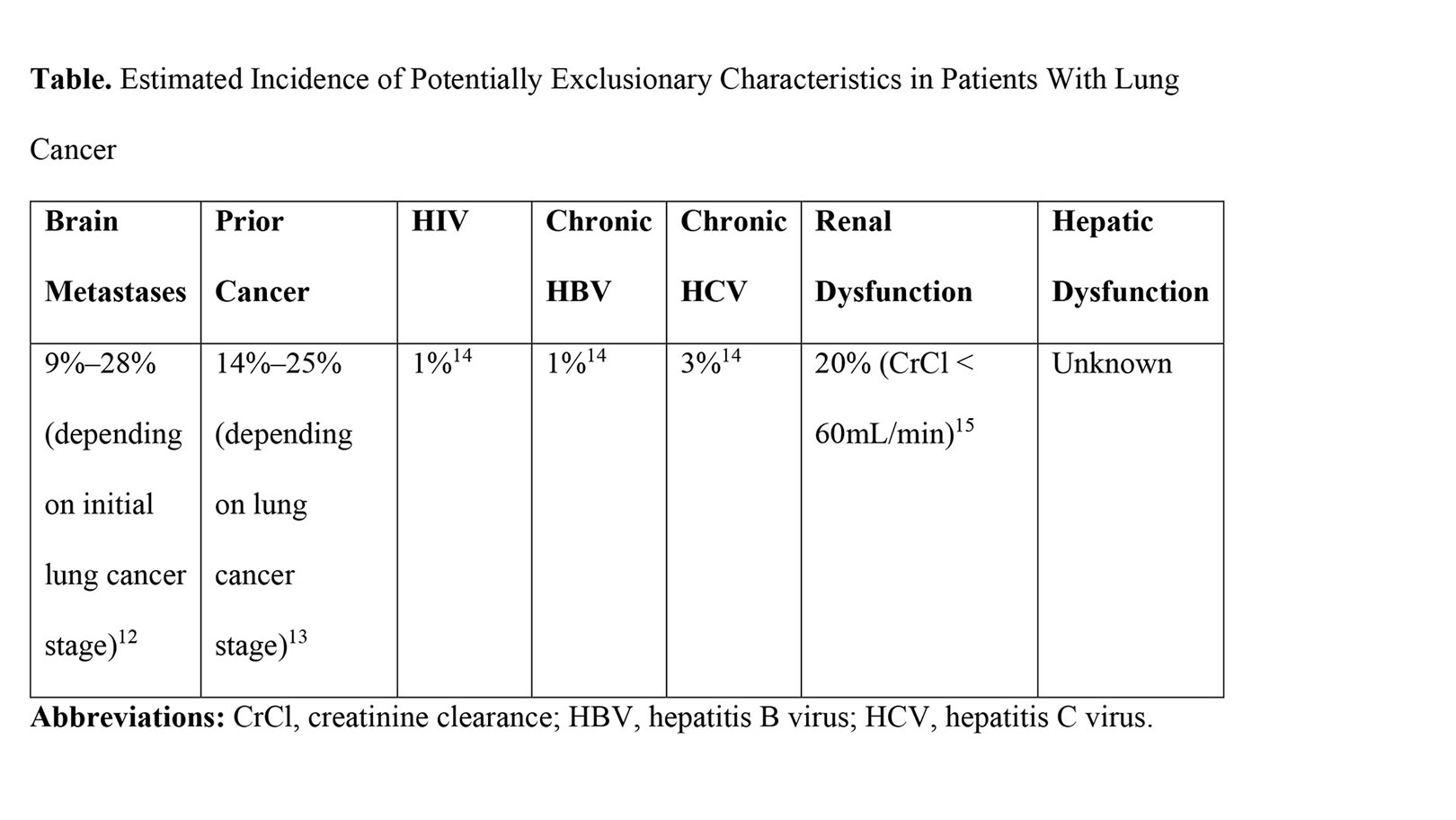
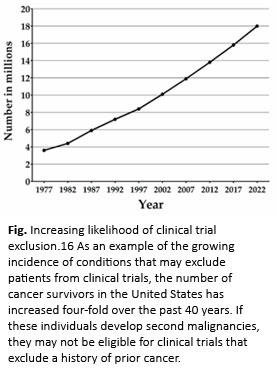
In July 2020, the U.S. Food and Drug Administration (FDA) revised its guidance on cancer clinical trial eligibility. The FDA guidance is comprehensive and thoughtful. If these recommendations are implemented, they are likely to increase clinical trial enrollment and completion. Intended to assist stakeholders—including sponsors and institutional review boards—responsible for the development and oversight of clinical trials, these recommendations address brain metastases, prior or concurrent malignancy, organ dysfunction, chronic viral infections, and minimum age requirements. Many of these topics have direct and increasing relevance to lung cancer populations (Table and Fig.). Below, we briefly summarize the content and review the potential implications of the new FDA guidance on lung cancer. Full details can be found at https://www.fda.gov/about-fda/oncology-center-excellence/oncology-center-excellence-guidance-documents.
Brain Metastases
Neurologically stable patients with treated/stable brain metastases should be included in trials unless there is a strong rationale to exclude them, regardless of whether study therapy is predicted to penetrate the blood–brain barrier. Inclusion of patients who have previously treated, stable brain metastases and who are on stable or decreasing doses of corticosteroids should be considered. Patients with active brain metastases should be included if deemed unlikely to require immediate central nervous system (CNS)–specific treatment, and if CNS activity of the study therapy is likely or CNS metastases are common in the target population. Patients with leptomeningeal metastases should be included if immediate CNS-specific treatment is unlikely to be required and the drug is anticipated to have CNS activity.
Nowhere are these recommendations poised to have a greater impact than for patients with lung cancer who have brain metastases. Lung cancer is the most common primary cancer to metastasize to the brain, with an overall incidence of 20% prior to widespread use of brain MRI scans, including 9% of individuals initially diagnosed with localized disease and 28% of patients presenting with metastatic disease.12 Brain metastases also occur relatively early in the clinical course of patients with lung cancer, at a median of 11 months, compared with 44 months for breast cancer.17 Additionally, patients with brain metastases have had meaningful improvement in outcomes — reflecting both earlier detection and better treatments — with survival more than doubling in the past 20 years.18 Over this same time period, reflecting the growing use of brain MRI scans, brain metastases are significantly more likely to be detected simultaneously with the primary tumor (30% vs. 18%) and significantly more likely to be multiple rather than solitary (60% vs. 30%).
Given these developments, the frequent requirement to perform pre-study brain imaging in patients without CNS symptoms, and growing numbers of systemic therapies with intracranial efficacy,19 there is little to no justification to exclude patients with brain metastases from clinical trials. In many instances, protocol requirements to treat brain metastases definitively prior to initiating study therapy may also be clinically inappropriate. By promoting the inclusion of patients with untreated, asymptomatic brain metastases in clinical trials, the FDA guidance is consistent with current clinical practice in multidisciplinary thoracic oncology programs, where medical oncologists and radiation oncologists frequently recommend initiation of systemic therapy and plan short-term repeat brain imaging. Furthermore, including the majority of patients with lung cancer who have brain metastases in clinical trials is likely to expedite the identification of effective treatments for CNS metastases and to increase trial accrual.
Prior or Concurrent Malignancy
Patients with prior or concurrent malignancies of the same or different tumor type whose natural history or treatment does not have the potential to interfere with safety or efficacy assessment should be eligible for enrollment in clinical trials.
While these recommendations leave the potential impact of another cancer diagnosis to interpretation, in lung cancer this issue has been studied in detail. Depending on the stage of the lung cancer, the likelihood of a prior cancer (of different type) ranges from 15% to 24%.18 It is important to note that more than half of these earlier malignancies occur within 5 years of the lung cancer diagnosis, the most common prior-cancer time period employed in trial-exclusion criteria. Regardless of the type, timing, or stage of a prior cancer, a prior cancer diagnosis does not appear to adversely affect lung cancer–specific survival or OS.14,15,19 Together, these observations suggest that permitting enrollment of individuals with prior malignancy into lung cancer clinical trials is likely to enhance enrollment and generalizability without compromising efficacy or safety.
Organ Dysfunction
As information on toxicity, pharmacokinetics, and pharmacodynamics becomes available during phase I studies, sponsors and investigators should enroll patients with compromised organ function and use these data to adjust doses of the experimental agent for these participants. Creatinine clearance rather than serum creatinine should be used to assess renal function. Consider including patients with National Cancer Institute Common Terminology Criteria for Adverse Events grade 3 aspirate aminotransferase and alanine aminotransferase elevations (greater than 5 to 20 times the upper limit of normal) who may be asymptomatic and able to tolerate drug doses equivalent to those of patients with normal hepatic function.
Mandating creatinine clearance of 60 mL/min or greater—a commonly used threshold in clinical trials—may exclude 20% of patients with lung cancer. Lowering the requirement to 30 mL/min or greater—an adequate threshold for many contemporary therapies, including some immune checkpoint inhibitors and molecularly targeted therapies—results in exclusion of fewer than 5% of patients.15 Therefore, basing renal function eligibility on known toxicity and metabolic profiles of experimental agents may enhance accrual without increasing risk.
HIV, Hepatitis B Virus (HBV), and Hepatitis C Virus (HCV)
For HIV, patients with CD4+ counts of 350 cells/μL or greater should generally be eligible for any study. Patients with CD4+ counts of less than 350 cells/μL should generally be eligible if they have a potentially curable malignancy or for interventions that have previously demonstrated HIV-beneficial activity. Patients should be on retroviral therapy, and HIV viral load should be less than 400 copies/mL. For chronic HBV and HCV, liver-related laboratory eligibility criteria should be the same as that for the general population. Because HBV reactivation can occur in chronic carriers or in individuals with resolved prior HBV infection, these patients should be on suppressive therapy prior to initiation of cancer treatment. In general, patients with HCV should have completed curative antiviral treatment and have undetectable HCV viral load. However, for incurable cancers, patients with untreated HCV may be enrolled if the HCV is stable and the investigational therapy is not expected to exacerbate the HCV infection.
Globally, there is high prevalence of HIV (33 million people) and viral hepatitis (380 million people). Furthermore, many clinical centers require viral hepatitis testing prior to initiating anti-cancer therapies, which could result in elevated rates in oncology populations. Indeed, among patients with cancer who have HIV, chronic HBV, or chronic HCV, 6%, 42%, and 31%, respectively, of cases are diagnosed through such screening.14 Based on literature reviews of patients with advanced cancer who have HIV, HBV, or HCV and who are treated with checkpoint inhibitors, it appears that toxicity and efficacy are comparable to patients without these viral infections.20,21 Although direct-acting antiviral therapy results in viral clearance in 90% of cases, HCV treatment takes up to 12 weeks, which is often impractical to complete prior to starting cancer treatment. Thus, the recommendation to consider enrollment of patients with untreated HCV in certain circumstances could have considerable effect on lung cancer clinical trials.
Minimum Age Requirement
Consider eligibility of pediatric populations when there is rationale and evidence to suggest benefit and justify patient risk.
This has, however, minimal relevance to lung cancer, which occurs in older adults and is exceedingly rare in children and adolescents.
Conclusion
The updated FDA guidance represents an important addition to the growing movement to reform the design and conduct of cancer clinical trials. Considering the incidence of brain metastases, creatinine clearance of less than 60 mL/min, and prior cancer, even if there is considerable overlap in these populations, relaxing these eligibility criteria is likely to expand access to trials substantially.
However, the FDA documents convey recommendations, not requirements. Investigators and sponsors who design and support clinical trials must be willing to revisit longstanding beliefs and practices. For years, the FDA has recommended greater use of remote trial monitoring to simplify and reduce costs of clinical research.22 Yet until the COVID-19 pandemic, few sponsors and contract research organizations employed this approach. This global health crisis has also introduced numerous other welcome adjustments to clinical trials, including remote consent, telehealth study visits, and shipping oral therapy to patients’ homes.23
But none of these changes influence trial eligibility. We hope that trial sponsors will be receptive to the collective voices of patients, their families and caregivers, advocacy groups and professional organizations such as the IASLC, investigators, and now a major regulatory authority, and that a meaningful change in clinical trial eligibility will occur.
References:
- Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720-2726.
- Ross S, Grant A, Counsell C, et al. Barriers to participation in randomised controlled trials: a systematic review. J Clin Epidemiol. 1999;52(12):1143-1156.
- Sertkaya A, Wong HH, Jessup A, et al. Key cost drivers of pharmaceutical clinical trials in the United States. Clin Trials. 2016;13(2):117-126.
- Kesselheim AS, Avorn J. New “21st Century Cures” legislation: speed and ease vs science. JAMA. 2017;317(6):581-582.
- Garcia S, Bisen A, Yan J, et al. Thoracic oncology clinical trial eligibility criteria and requirements continue to increase in number and complexity. J Thorac Oncol. 2017;12(10):1489-1495.
- Gerber DE, Lakoduk AM, Priddy LL, et al. Temporal trends and predictors for cancer clinical trial availability for medically underserved populations. Oncologist. 2015;20(6):674-682.
- Hardy D, Liu CC, Xia R, et al. Racial disparities and treatment trends in a large cohort of elderly black and white patients with nonsmall cell lung cancer. Cancer. 2009;115(10):2199-2211.
- Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early-stage lung cancer. N Engl J Med. 1999;341(16):1198-1205.
- Avis NE, Smith KW, Link CL, et al. Factors associated with participation in breast cancer treatment clinical trials. J Clin Oncol. 2006;24(12):1860-1867.
- Forde PM, Bonomi P, Shaw A, et al. Expanding access to lung cancer clinical trials by reducing the use of restrictive exclusion criteria: perspectives of a multistakeholder working group. Clin Lung Cancer. 2020;21(4):295-307.
- Kim ES, Bruinooge SS, Roberts S, et al. Broadening eligibility criteria to make clinical trials more representative: American Society of Clinical Oncology and Friends of Cancer Research joint research statement. J Clin Oncol. 2017;35(33):3737-3744.
- Barnholtz-Sloan JS, Sloan AE, Davis FG, et al. Incidence proportions of brain metastases in patients diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J Clin Oncol. 2004;22(14):2865-2872.
- Gerber DE, Laccetti AL, Xuan L, et al. Impact of prior cancer on eligibility for lung cancer clinical trials. J Natl Cancer Inst. 2014;106(11):dju302.
- Ramsey SD, Unger JM, Baker LH, et al. Prevalence of hepatitis B virus, hepatitis C virus, and HIV infection among patients with newly diagnosed cancer from academic and community oncology practices. JAMA Oncol. 2019;5(4):497-505.
- Lichtman SM, Harvey RD, Damiette Smit MA, et al. Modernizing clinical trial eligibility criteria: recommendations of the American Society of Clinical Oncology-Friends of Cancer Research organ dysfunction, prior or concurrent malignancy, and comorbidities working group. J Clin Oncol. 2017;35(33):3753-3759.
- De Moor JS, Mariotto AB, Parry C, et al. Cancer survivors in the United States: prevalence across the survivorship trajectory and implications for care. Cancer Epidemiol Biomarkers Prev. 2013;22:561-570.
- Berghoff AS, Schur S, Fureder LM, et al. Descriptive statistical analysis of a real life cohort of 2419 patients with brain metastases of solid cancers. ESMO Open. 2016;1(2):e000024.
- Nieder C, Spanne O, Mehta MP, et al. Presentation, patterns of care, and survival in patients with brain metastases: what has changed in the last 20 years? Cancer. 2011;117(11):2505-2512.
- Achrol AS, Rennert RC, Anders C, et al. Brain metastases. Nat Rev Dis Primers. 2019;5(1):5.
- Cook MR, Kim C. Safety and efficacy of immune checkpoint inhibitor therapy in patients with HIV infection and advanced-stage cancer: a systematic review. JAMA Oncol. 2019;5(7):1049-1054.
- Shah NJ, Al-Shbool G, Blackburn M, et al. Safety and efficacy of immune checkpoint inhibitors (ICIs) in cancer patients with HIV, hepatitis B, or hepatitis C viral infection. J Immunother Cancer. 2019;7(1):353.
- U.S. Department of Health and Human Services, Food and Drug Administration. Guidance for industry: oversight of clinical investigations—a risk-based approach to monitoring. 2013. OMB Control No. 0910-0733.
- de Paula BHR, Araujo I, Bandeira L, et al. Recommendations from national regulatory agencies for ongoing cancer trials during the COVID-19 pandemic. Lancet Oncol. 2020;21(5):624-627.