The Lung Master Protocol (Lung-MAP; S1400) was a biomarker-driven master protocol. This trial was originally designed to address the relative paucity of effective, targeted therapies in squamous cell lung cancer, compared to non-squamous NSCLC subtypes.1
This trial was initiated in 2014, when the standard-of-care treatment for patients with advanced squamous cell lung cancer consisted of platinum-doublet chemotherapy in the first line, followed by several potential single-agent cytotoxic options in the second line and beyond. No targeted therapies were available. At that time, and still today, there was a need to improve our understanding of the genomic landscape of squamous cell lung cancer to find better targets. Advances in broad-based, next-generation sequencing (NGS) technologies enabled us to identify several relatively rare mutations, occurring in 5% to 15% of patients, as potential therapeutic targets.2
,3
,4
Because of the rarity of these potential driver mutations, an umbrella protocol was designed to study multiple investigational therapies matched to biomarkers within a single clinical trial infrastructure. This allowed for accelerated testing of targeted therapeutic agents, which received immediate support from the National Cancer Institute, the U.S. Food and Drug Administration (FDA), academic researchers, and patient advocacy groups.
The primary objective of this study was to establish an infrastructure that could be used to efficiently evaluate targeted therapies in biomarker subgroups. The infrastructure created allowed for biomarker testing and evaluation of targeted therapies with regulatory intent. Patients included in this trial had stage IV or recurrent squamous cell lung cancer and had received previous platinum-doublet chemotherapy. The study design is summarized in the figure. The screening component of the trial allowed for organized specimen collection and broad-based NGS for all patients. The clinical trial component assigned patients to biomarker-driven substudies if they had alterations of PIK3CA (taselisib), cell cycle gene (palbociclib), FGFR (AZD4547), homologous recombination repair (talazoparib), or MET (rilotumumab plus erlotinib or telisotuzumab vedotin). The comparator arms varied; PIK3CA, cell cycle gene, and FGFR substudies were compared to standard-of-care docetaxel. The MET-matched substudy of rilotumumab plus erlotinib was compared to erlotinib. The other MET-matched substudy of telisotuzumab vedotin as well as the homologous recombination repair–matched substudies were single arm. If patients did not have any of these biomarkers, then they were assigned to one of the nonmatched substudies evaluating durvalumab (S1400A versus standard-of-care docetaxel) or, later, nivolumab plus ipilimumab (versus standard-of-care nivolumab) for checkpoint inhibitor–naive disease, and durvalumab plus tremelimumab (single-arm study) for checkpoint inhibitor–relapsed disease. Appropriate protocol changes were made in 2015 upon FDA approval of nivolumab in the second-line setting, replacing docetaxel as standard of care.5
,6
The docetaxel arm was closed for the matched substudies targeting alterations of PIK3CA, cell cycle gene, and FGFR (S1400B-D) and the nonmatched substudy utilizing single-agent durvalumab (S1400A), and each was modified from a phase II/III to a single-arm phase II design. The nonmatched substudy for checkpoint inhibitor–naive disease with nivolumab plus ipilumumab versus nivolumab was initiated at that time. Of note, the MET alteration matched substudy of rilotumumab plus erlotinib versus erlotinib was discontinued in November 2014 because of ceased development of rilotumumab.
Lung-MAP S1400 successfully established the infrastructure to implement the first biomarker-driven master protocol within the National Cancer Institute. This study demonstrated that comprehensive, centralized, genomic testing with commercially available NGS was feasible. Additionally, biomarker-driven studies, with a nonmatched option, could be carried out simultaneously with rare genotypes to efficiently assess targeted drug activity. Unfortunately, the targeted therapies investigated did not show clinical activity.1
,7
,8
,9
,10
,11
,12
,13
However, Lung-MAP S1400 allowed for collection of broad-based NGS in “real-world” patients with squamous cell lung cancer with annotated clinical data. This provided a platform to conduct novel retrospective and translational biomarker studies in this population. The genomic data obtained also highlight the importance of broad-based molecular sequencing for all patients with advanced squamous cell lung cancer. Without a better understanding of the genomic landscape, we will never be able to distinguish potential targets, develop new biomarker-driven therapies, or improve outcomes in this mutationally complex disease.
One limitation of this study was that we were unable to differentiate biomarkers as being prognostic or predictive. Biomarker studies were performed in biomarker-matched patient subgroups; thus, data outside of the trial are needed to interpret potentially predictive biomarkers. Another major challenge during this study was the rapid change in the treatment landscape of NSCLC with the advent of immunotherapy. The trial was efficiently updated to change eligibility criteria, statistical design, and the option to prescreen patients during first-line treatment. This highlights the importance that biomarker-driven protocols be adaptable, as well as the necessity of effective communication to make any changes seamless across all subgroups.
There remains an unmet need to identify the most effective therapy in the second-line space, especially with the FDA approval of front-line chemoimmunotherapy, for both squamous and non-squamous NSCLC. This protocol has been revised to address this important question by evaluating personalized, biomarker-driven therapies (S1800/S1900; see Fig.) in both histologies. Dynamic umbrella studies such as this will continue to enhance our understanding of the mutational landscape and advance individualized lung cancer therapies.
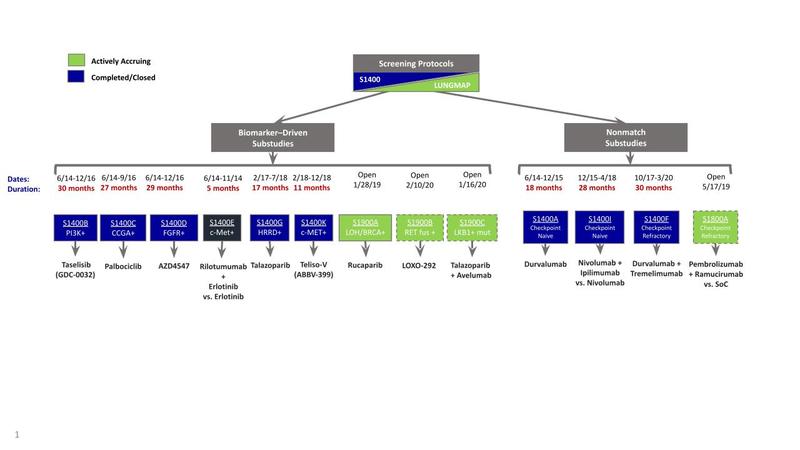
Abbreviations: CCGA, circulating cell-free genome atlas; fus, fusion; HRRD, homologous recombination repair deficiency; LOH, loss of heterozygosity; mut, mutation; SoC, standard of care; teliso-v, telisotuzumab vedotin.
- 1. a. b. Redman MW, Papadimitrakopoulou VA, Minichiello K, et al. Biomarker-driven therapies for previously treated squamous non-small-cell lung cancer (Lung-MAP SWOG S1400): a biomarker-driven master protocol. Lancet Oncol. 2020;21(12):1589-1601.
- 2. Gandara DR, Hammerman PS, Sos ML, Lara PN Jr, Hirsch FR. Squamous cell lung cancer: from tumor genomics to cancer therapeutics. Clin Cancer Res. 2015;21(10):2236-2243.
- 3. Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489(7417):519-525. Published correction appears in Nature. 2012;491(7423):288.
- 4. Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023-1031.
- 5. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373(2):123-135.
- 6. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639.
- 7. Borghaei H, Redman MW, Kelly K, et al. SWOG S1400A (NCT02154490): a phase II study of durvalumab for patients with previously treated stage IV or recurrent squamous cell lung cancer (Lung-MAP sub-study). Clin Lung Cancer. 2020. [Epub ahead of print].
- 8. Langer CJ, Redman MW, Wade JL 3rd, et al. SWOG S1400B (NCT02785913), a phase II study of GDC-0032 (taselisib) for previously treated PI3K-positive patients with stage IV squamous cell lung cancer (Lung-MAP sub-study). J Thorac Oncol. 2019;14(10):1839-1846.
- 9. Edelman MJ, Redman MW, Albain KS, et al. SWOG S1400C (NCT02154490)—a phase II study of palbociclib for previously treated cell cycle gene alteration-positive patients with stage IV squamous cell lung cancer (Lung-MAP substudy). J Thorac Oncol. 2019;14(10):1853-1859.
- 10. Aggarwal C, Redman MW, Lara PN Jr, et al. SWOG S1400D (NCT02965378), a phase II study of the fibroblast growth factor receptor inhibitor AZD4547 in previously treated patients with fibroblast growth factor pathway-activated stage IV squamous cell lung cancer (Lung-MAP substudy). J Thorac Oncol. 2019;14(10):1847-1852.
- 11. Owonikoko TK, Redman MW, Byers LA, et al. Phase 2 study of talazoparib in patients with homologous recombination repair-deficient squamous cell lung cancer: Lung-MAP substudy S1400G. Clin Lung Cancer. 2021. [Epub ahead of print].
- 12. Waqar SN, Redman MW, Arnold SM, et al. A phase II study of telisotuzumab vedotin in patients with c-MET-positive stage IV or recurrent squamous cell lung cancer (LUNG-MAP sub-study S1400K, NCT03574753). Clin Lung Cancer. 2020. [Epub ahead of print].
- 13. Bazhenova L, Redman MW, Gettinger SN, et al: A phase III randomized study of nivolumab plus ipilimumab versus nivolumab for previously treated patients with stage IV squamous cell lung cancer and no matching biomarker (Lung-MAP Sub-Study S1400I, NCT02785952). Journal of Clinical Oncology 37:9014-9014, 2019






