Use of platinum-doublet chemotherapy regimen as second-line treatment for patients with relapsed small-cell lung cancer (SCLC) was a reasonable option compared with nonplatinum-based regimens, according to data from a meta-analysis presented at the WCLC Congress 2021.
“Optimal second-line chemotherapy for patients with relapsed SCLC remains unclear,” according to study presenter Takashi Sato, MD, PhD, of Kitasato University School of Medicine, Sagamihara, Japan.
One recent randomized controlled trial by the French Lung Cancer Group compared topotecan and platinum-etoposide for patients with sensitive, relapsed SCLC and showed significantly longer progression-free survival (PFS) in the platinum-doublet chemotherapy group compared with topotecan.1 With a median follow-up of 22.7 months, the median PFS was 4.7 months compared with 2.7 months.
To look at this topic further, this meta-analysis included studies that enrolled patients with relapsed SCLC and compared platinum-doublets with nonplatinum-based regimens in the second line. Based on these criteria, 10 studies published from 2011 to 2020 were included; these included 1,222 patients (438 treated with platinum doublets). There were seven retrospective studies, one prospective cohort, and two randomized controlled trials.
Median PFS was available for seven studies and ranged from 3.6 months to 6.2 months in the platinum-doublet groups and from 2.3 months and 5.4 months in the nonplatinum-based treatment groups. Median overall survival was available for six studies. This ranged from 8.0 months to 14.3 months for those who received platinum-doublet therapy and from 5.2 months to 14.4 months for those who received nonplatinum-based therapy.
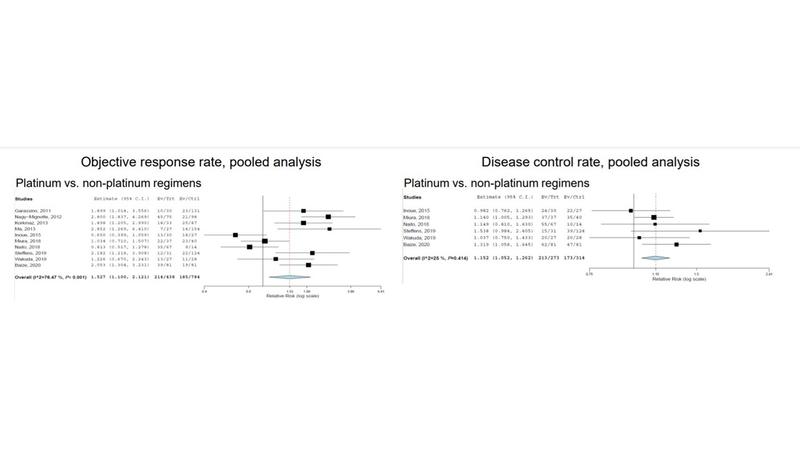
The researchers calculated pooled objective response rates (ORR) and disease control rates. Patients treated with platinum doublets had higher ORR compared with nonplatinum-based regimens (relative risk [RR] = 1.527; 95% CI: 1.1-2.121; P < 0.001). A subgroup analysis comparing platinum doublets with topotecan or amrubicin also showed a higher ORR among all patients (RR = 1.663; 95% CI: 1.055-2.619; P < 0.001) and those with sensitive relapsed disease (RR = 1.247; 95% CI: 0.836-1.860; P = 0.019).
Use of platinum doublet also resulted in a trend toward better disease control rate compared with nonplatinum-based regimens (RR = 1.152; 95% CI: 1.052-1.262; P = 0.414), and in subgroup analysis compared with topotecan or amrubicin (RR = 1.170; 95% CI: 1.021-1.340; P = 0.189) and sensitive disease (RR = 1.143; 95% CI: 1.024-1.275; P = 0.339).
According to study discussant, Arish Thomas, MD, an investigator with the National Cancer Institute, Bethesda, Maryland, there are several major challenges in SCLC that make it a challenging cancer to treat including chemotherapy resistance, lack of clinically relevant molecular subgroups, and intratumor heterogeneity.
When discussing this meta-analysis, Dr. Thomas noted that the inclusion of so many retrospective studies opened the meta-analysis to selection bias. He also noted the variability of agents combined with platinum-based chemotherapies in the included studies.
Dr. Thomas agreed that the general direction of the pooled analysis favored platinum compared with nonplatinum regimens. However, he noted that the magnitude of this benefit was unclear as was the clinical benefit.
These tumors may respond to more platinum, Dr. Thomas said, but eventually they will become more resistant, and patients will eventually succumb to the disease. Novel approaches to re-sensitize resistant tumors to chemotherapy are needed.
- 1. Baize N, Monnet I, Greillier L, et al. Carboplatin plus etoposide versus topotecan as second-line treatment for patients with sensitive relapsed small-cell lung cancer: an open-label, multicentre, randomized, phase 3 trial. Lancet Oncol. 2020;21(9):1224-1233.